Abstract
Chronic renal failure (CRF) is associated with oxidative stress that promotes production of reactive oxygen species and cytokine release. We aimed to investigate the possible protective and antioxidant effects of aqueous garlic extract (AGE) in a rat model of CRF. Male Sprague-Dawley rats were randomly assigned as either CRF group with 5/6 reduction in the renal mass or sham-operated control group. CRF group received either saline or AGE (250 mg/kg/day/1 mL) orally for 3 weeks. At the end of the 3 weeks, rats were decapitated and trunk blood was collected. Creatinine, blood urea nitrogen (BUN) and lactate dehydrogenase (LDH) activity, and TNF-α and IL-1β levels were measured in the serum samples, while malondialdehyde (MDA), glutathione (GSH) levels, and myeloperoxidase (MPO) activity were determined in the kidney, lung, and heart samples. CRF caused significant decreases in tissue GSH, which were accompanied with significant increases in MDA levels and MPO activities, while the circulating levels of the LDH activity, creatinine, BUN, TNF-α, and IL-1β were elevated. AGE treatment alleviated CRF-induced oxidative changes in the injured tissues, while CRF-induced elevations in the blood levels of the pro-inflammatory cytokines and LDH were reduced. In conclusion, CRF-induced oxidative tissue injury occurs via the activation of pro-inflammatory mediators and by neutrophil infiltration into tissues and that the protective effects of garlic on CRF-induced injury can be attributed to its ability to inhibit neutrophil infiltration and pro-inflammatory mediators. These findings suggest that garlic, as a supplementary to diet, may have a potential therapeutic use in delimitating the systemic oxidant effects of CRF on remote organs.
INTRODUCTION
The morbidity and mortality associated with chronic renal failure (CRF) are primarily caused by atherosclerosis, which may be in part caused by oxidative stress. Oxidative stress was shown to be increased in patients with renal impairment as a result of increased oxidant activity and reduced antioxidant capacity, and this was increased in a graded manner with increasing renal dysfunction.Citation1,2 In the early phase of CRF, the presence of oxidative stress was verified by increased plasma 8-isoprostane levels, while other markers of oxidative stress [malondialdehyde (MDA), glutathione (GSH) peroxidase (GPx), and oxidized serum albumin] measured during the later phases indirectly confirmed that oxidative stress is especially important in renal dysfunction.Citation3,4 In CRF, the imbalance between increased production of reactive oxygen species (ROS) and subsequent cytokine release along with limited or decreased antioxidant capacity results in a persistent formation of toxic lipid peroxidation products,Citation5,6 damaging the organic molecules such as proteins, nucleic acids, and lipids, contributing to the development of serious complications in CRF.Citation7,8 In patients with CRF, activation of polymorphonuclear neutrophils is a well-recognized feature with documented association between renal dysfunction and the different mediators and markers of inflammation such as interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), and fibrinogen.Citation9 Chronic inflammatory response is a highly prevalent co-morbid condition that predicts poor clinical outcome in CRF patients, increasing mortality.Citation10
Garlic (Allium sativum L.), which is an important and widely cultivated plant with both culinary and medicinal uses, has been used as a folk remedy for a variety of ailments since ancient times.Citation11 In several animal models, it has been demonstrated that garlic preparations prevented tumor promotion and liver damage and delayed the progression of cardiovascular diseases and aging, which are considered to be associated with oxygen radical generation and lipid peroxidation.Citation12–15 Garlic-derived allium derivatives have been shown to exert antibiotic, anticancer, antithrombotic, and lipid-lowering cardiovascular effects.Citation16 Garlic and other members of the Allium family are unusual in containing very high levels of organic sulfur compounds, and many of the reported beneficial effects of these vegetables have been attributed to these organic sulfur compounds.Citation16 The intrinsic antioxidant activity of garlic extracts and its constituents has been widely documented using in vivo and in vitro experimental models.Citation17–19 Garlic extracts increased superoxide dismutase (SOD), GPx, and catalase (CAT) activities in cultured vascular cells,Citation20,21 while a garlic compound, alliin, prevented the decrease in hepatic SOD and CAT activities in diabetic rats.Citation19,21
Garlic extract and its various components were postulated to have an important cytoprotective role in the setting of ischemia/reperfusion (I/R) injury through their antioxidant and anti-inflammatory properties. Accordingly, we have previously studied the protective and antioxidant effects of garlic in I/R-induced hepatic, cerebral, and renal injury.Citation22–24 Based on the aforementioned observations, the aim of the present study was to elucidate the possible protective effects of aqueous garlic extract (AGE) on CRF-induced renal, cardiac, and pulmonary injury by using biochemical and histopathological analyses.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (220–250 g), supplied by the Marmara University (MU) Animal Center (DEHAMER), were kept at a constant temperature of 22 ± 2°C with light–dark cycles of 12 h. Rats were fed a standard diet and water ad libitum. Experimental protocols were approved by the MU Animal Care and Use Committee.
Surgery and Experimental Design
The rats that were randomly assigned to either the CRF (n = 16) or the sham-operated control groups (n = 8) were anesthetized with ketamine (100 mg/kg) and chlorpromazine (0.75 mg/kg) given intraperitoneally. In order to induce CRF, rats underwent 5/6 nephrectomy by surgical resection of the upper and lower thirds of the left kidney, followed by right nephrectomy.Citation25 The rats in the sham-operated control group had similar anesthetic procedures and bilateral dorsal incisions without any surgical resections.
CRF group received either AGE (250 mg/kg/day, n = 8) in 1 mL or saline (1 mL; n = 8) by gavage for 3 weeks. One milliliter of AGE contained material from 500 mg of garlic, prepared from peeled garlic crushed with distilled water in a mortar. The dose and preparation of AGE were based on our previous reports.Citation24,26 At the end of the 3 weeks, rats were decapitated and trunk blood was collected. Blood urea nitrogen (BUN) and creatinine levels were determined as indicators of kidney function. Serum lactate dehydrogenase (LDH) activity was measured as a marker of systemic tissue injury. TNF-α and IL-1β levels were also measured in the serum samples, while the tissue samples from the kidney, heart, and lung were immediately taken and stored at –80°C. In all tissue samples, MDA and GSH levels and tissue-associated myeloperoxidase (MPO) activity were determined.
Biochemical Analysis
BUN,Citation27 serum creatinine,Citation28 and serum LDH levelsCitation29 were determined spectrophotometrically using an automated analyzer. Serum levels of TNF-α and IL-1β were quantified according to the manufacturer’s instructions and guidelines using enzyme-linked immunosorbent assay (ELISA) kits specific for the previously mentioned rat cytokines (Biosource International, Nivelles, Belgium). These particular assay kits were selected because of their high degree of sensitivity, specificity, inter- and intra-assay precision, and small amount of serum sample required for conducting the assay.
Measurement of Tissue Myeloperoxidase Activity
Tissue-associated MPO activity, which is accepted as an indicator of neutrophil infiltration in tissues, was measured by a procedure similar to that described by Hillegas et al.Citation30 Kidney, heart, and lung samples in 0.2–0.3 g were homogenized in 10 volumes of ice-cold potassium phosphate buffer (20 mM K2HPO, pH 7.4). The homogenate was centrifuged at 12,000 rpm (41400 × g) for 10 min at 4°C, and the supernatant was discarded. The pellet was then rehomogenized with an equivalent volume of 50 mM K2HPO containing 0.5% (wt/vol) hexadecyltrimethylammonium hydroxide (Sigma, Saint Louis, MO, USA). MPO activity was assessed by measuring the H2O2-dependent oxidation of o-dianisidine·2HCl. One unit of enzyme activity is defined as the amount of the MPO present that causes a change in absorbance of 1.0 min−1 at 460 nm and 37°C.Citation31
Measurement of Tissue Malondialdehyde and Glutathione Levels
For the measurement of MDA and GSH, renal, cardiac, and pulmonary tissue samples were homogenized in 10 volumes of ice-cold 10% trichloroacetic acid and centrifuged at 3000 rpm (2000 × g) for 15 min at 4°C. Supernatant was removed and recentrifuged at 10,000 rpm at 4°C for 8 min. The supernatant was transferred to a test tube containing an equal volume of thiobarbituric acid (TBA; 0.67% w/v), and this mixture was then heated to 90°C and maintained at that temperature for 15 min. The MDA concentration for each specimen was determined in a spectrophotometer based on the level of absorbance at 532 nm and was expressed as nmol/g tissue.Citation32 GSH measurements were performed using a modification of the Ellman procedure.Citation33 Briefly, after centrifugation at 3000 rpm (2000 × g) for 10 min, 0.5 mL supernatant was added to 2 mL 0.3 mol/L Na2HPO4·2H2O solution. A 0.2 mL solution of dithiobisnitrobenzoate (0.4 mg/mL 1% sodium citrate) was added and the absorbance at 412 nm was measured immediately after mixing. GSH levels were calculated using an extinction coefficient of 1.36 × 105 M/cm. Results are expressed in μmol GSH/g tissue.
Table 1. Serum levels of blood urea nitrogen (BUN), creatinine, lactate dehydrogenase (LDH), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β) in the sham-operated control and saline- or aqueous garlic extract (AGE)-treated chronic renal failure (CRF) groups. For each group n = 8.
Histopathological Analysis
For light microscopic investigations, tissue specimens were fixed in 10% formaldehyde, dehydrated in increasing alcohol series, cleared in toluene, and embedded in paraffin. Paraffin sections (5 μm) were stained with hematoxylin and eosin (H&E) and examined under a photomicroscope (Olympus BX51, Tokyo, Japan) for the characterization of histopathological changes by an experienced histologist (FE) in a blinded fashion.
Statistics
Statistical analysis was done using a Graph Pad Prism 3.0 (Graph Pad Software, San Diego, CA, USA). All data are expressed as means ± SEM. Groups of data were compared with an analysis of variance (ANOVA) followed by Turkey’s multiple comparison tests. Values of p < 0.05 were considered as significant.
RESULTS
Serum BUN and creatinine levels, assessed to evaluate the severity of CRF, were significantly higher in the saline-treated CRF group than those of the control group (p < 0.001; ). AGE treatment significantly reduced (p < 0.001) the increase in BUN level, while the elevation in serum creatinine level was abolished in AGE-treated CRF group (p < 0.01). When compared to the sham-operated control group, in the saline-treated rats with CRF, serum levels of LDH, as well as pro-inflammatory cytokines, TNF-α and IL-1β were significantly increased as markers of systemic tissue injury (p < 0.01–0.001). On the other hand, CRF-induced elevation in LDH, TNF-α, and IL-1β levels was significantly reduced in the animals treated with the garlic extract (p < 0.05–0.01).
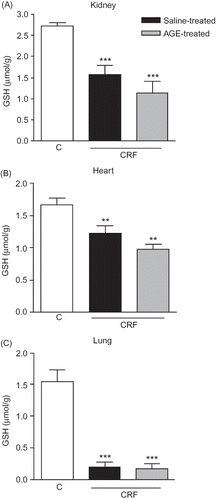
MPO activity, which is accepted as an indicator of neutrophil infiltration, was significantly increased in the renal, cardiac, and pulmonary tissues at the third week of renal failure (p < 0.05–0.01; ). However, AGE treatment throughout the 3-week CRF period significantly reduced the MPO activity in the renal tissue (p < 0.05), but increased MPO activity in both the heart and lung as CRF was abolished by the garlic extract (p < 0.01 and 0.001). The MDA levels, measured as a major degradation product of lipid peroxidation in the renal, cardiac, and pulmonary tissues, were found to be significantly higher in the saline-treated CRF group as compared to those of the control group (p < 0.01, ). Treatment with the AGE reduced the MDA levels in the heart and lung significantly (p < 0.05), but the AGE treatment did not significantly change increased lipid peroxidation of the renal tissue with chronic failure. Supporting the MDA and MPO findings, in the saline-treated CRF group, levels of the major cellular antioxidant GSH in the studied tissues were significantly decreased (p < 0.01–0.001, ). However, garlic treatment throughout the 3-week CRF period had no significant effect on the GSH levels of none of the tissues.
Figure 1. Myeloperoxidase (MPO) activity in the (A) kidney, (B) heart, and (C) lung tissues of sham-operated control, and saline- or aqueous garlic extract (AGE)-treated chronic renal failure (CRF) groups.
Notes: *p < 0.05, **p < 0.01; compared to control group.
+p < 0.05, ++p < 0.01, +++p < 0.01; compared to saline-treated CRF groups.
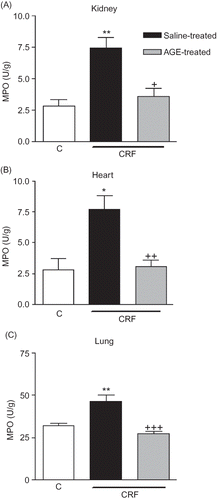
Figure 2. Malondialdehyde (MDA) levels in the (A) kidney, (B) heart, and (C) lung tissues of sham-operated control, and saline- or aqueous garlic extract (AGE)-treated chronic renal failure (CRF) groups.
Notes: *p < 0.01, **p < 0.01; compared to control group.
+p < 0.05, compared to saline-treated CRF group.
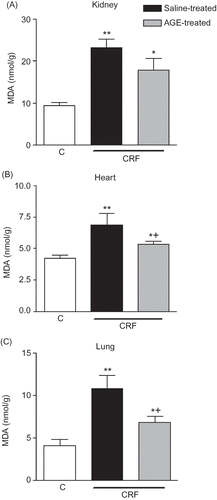
Figure 3. Glutathione (GSH) levels in (A) kidney, (B) heart, and (C) lung tissues of sham-operated control, and saline- or aqueous garlic extract (AGE)-treated chronic renal failure (CRF) groups.
Notes: *p < 0.01, **p < 0.01; compared to control group.
+p < 0.05, compared to saline-treated CRF group.
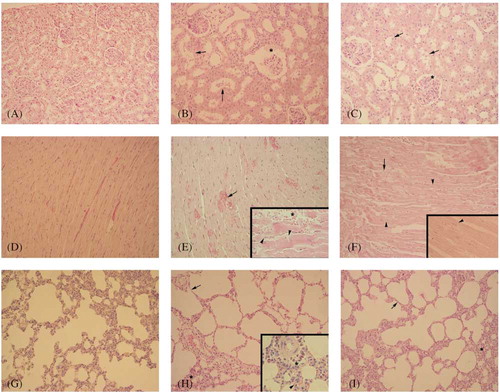
As compared to the sham-operated control group, with regular morphology of the kidney (), in the saline-treated CRF group () degenerated glomerular and Bowman’s space structures and severe degeneration in tubular epithelium with cellular debris in lumen of the tubules were observed. The cardiac tissue of rats with renal failure () demonstrated severe vascular congestion, hemorrhage in some regions, and degeneration in muscle fibers, while the regular morphology of the cardiac tissue was observed in the sham-operated control group (). With respect to lung tissue of the control rats (), the pulmonary tissue () of the rats with CRF showed severe inflammatory cell infiltration and alveolar degeneration. Histological analysis also revealed that degenerations in the kidney, heart, and lung tissues of the rats were clearly improved when the animals with CRF were treated by AGE ( C, F, and I).
DISCUSSION
Significant reduction of nephron mass by subtotal nephrectomy in animals or by disease processes in humans leads to CRF.Citation34 Following subtotal nephrectomy in the rats, a progressive deterioration of the renal functions takes place in the remnant kidney mediated by hemodynamic events as well as nonhemodynamic factors, such as oxidative stress and inflammation.Citation35 As observed by increased lipid peroxidation and MPO activity with a concomitant decrease in GSH levels, the present results demonstrate that CRF resulted in oxidative injury of the renal, as well as pulmonary and cardiac tissues. However, AGE treatment alleviated CRF-induced oxidative changes in the injured tissues, while CRF-induced elevations in the blood levels of the pro-inflammatory cytokines and LDH were reduced. These findings suggest that AGE, by inhibiting neutrophil infiltration and subsequent release of inflammatory mediators that induce lipid peroxidation, appears to play a protective role in CRF-induced oxidative injury of the renal and cardiopulmonary tissues.
The members of the Allium family, including garlic, contain very high levels of biochemically active organic sulfur compounds, and their extracts have been known to protect organs from various injuries.Citation36–38 Since ROS have been implicated in mediating various pathological processes (e.g., ischemic heart disease, peripheral arterial occlusive disease, hypertension, hyperlipidemia, and toxicities), it can be suggested that the beneficial effects of garlic in these conditions may be due to its antioxidant activity.Citation39–46 The compounds in garlic (S-allyl-cysteine, S-allyl-mercapto cysteine, S-allyl-cysteine sulfoxide, and allicin), through their radical scavenging abilities, have been suggested to be responsible for its preventive effects against oxidative injury.Citation14 Diallyldisulfide (DADS), a metabolite of allicin, includes redox-active sulfhydryl (SH)- or disulfide (-S-S)- groups which have already been proven to act as radical scavengers.Citation47 Aged garlic extract and diallyl polysulfides inhibit the formation of TBA-reactive substances and fluorescent substances induced by iron-ascorbic acid in isolated liver microsomal membranes, indicating the protection against lipid peroxidation.Citation37 Alliin scavenges the hydroxyl radical (.OH) and garlic powder scavenges both .OH and 1,1-diphenyl-2-picrylhydrazyl radicals.Citation37 Similarly, Yamasaki et al.Citation48 have shown that aged garlic extract protected vascular endothelial cells from H2O2-induced oxidative damage by inhibiting lipid peroxidation. It was also shown that aged garlic extract attenuated ischemic brain damage in the rat, and the garlic compounds, S-allyl cysteine, allyl sulfide, and allyl disulfide, were found to be responsible for this protective effect.Citation49 To our knowledge no study has studied the effect of garlic in a renal failure model so far, except for I/R studies.Citation16,22–24,50 In the current study, the protective effect of AGE against CRF, which is associated with oxidative stress promoting the production of ROS and cytokine release, was studied. The current findings revealed that CRF-induced increases in renal and cardiopulmonary MDA levels, indicating lipid peroxidation and oxidative damage,Citation51 were ameliorated when the animals were treated with AGE. Similarly, AGE was shown to exert cytoprotective effects in different models of experimental studiesCitation16 by preventing increases in MDA levels and preserving normal tissue morphology in rat kidney, liver, intestine, lung, and urinary bladder.Citation16,26,52 In the present study, AGE treatment significantly decreased MDA levels in the kidney, heart, and lung of CRF rats, probably in part, by scavenging the very reactive hydroxyl and peroxyl radicals. Horie et al.Citation38 suggested that AGE and diallyl polysulfides inhibit the formation of TBA-reactive and fluorescent substances induced by iron-ascorbic acid in isolated liver microsomal membranes, indicating the protective effect of garlic against lipid peroxidation. Similarly, it was previously shown in several nephrotoxicity models that oxidative damage in the kidney tissue was prevented by AGE, which was demonstrated by reduced MDA levels and restored renal functions.Citation22,23,26,46,53,54
Figure 4. Regular morphology of kidney (A), heart (D), and lung (G) tissues was observed in control group. Degenerated Bowman’s space and glomerular structure (*), and degenerated tubular epithelium and cell debris in tubular lumen (arrow) in kidney (B); severe vascular congestion (arrow), hemorrhage in some region (*), and degeneration in cardiac fibers (arrow head) in heart (E); alveolar disturbance (arrow), severe inflammatory cell infiltration (*), and vascular edema in lung (H) were observed in the saline-treated chronic renal failure (CRF) group. Quite regular Bowman’s space and glomerular structure (*), moderate degeneration in tubular epithelium (arrow), in kidney (C), mild degeneration in cardiac muscle fibers (arrow head) and mild vascular congestion (arrow) in heart (F), quite regular alveolar wall (arrow), and mild inflammatory cell infiltration (*) in lung tissues were observed in garlic-treated CRF group. Hematoxylin and eosin (H&E) staining, original magnifications: 200×; insets: 400×.
Oxidative stress-induced tissue damage can be prevented or ameliorated by favoring the balance toward a lower oxidative status. GSH provides major protection in oxidative injury by participating in the cellular defense against oxidative damage and protects protein thiol groups from oxidation,Citation55 while depletion of tissue GSH is one of the primary factors that permit lipid peroxidation to occur.Citation56 In the present study, tissue GSH levels were depleted in CRF animals. However, the AGE treatment, which ameliorated oxidative damage and cytokine levels, did not replenish GSH levels. It may be speculated that the lipid peroxidation and subsequent tissue injury were alleviated upon the expense of GSH stores, suggesting that the increased GSH by the stimulatory effect of garlic may have been used up to protect against oxidative injury. It was previously reported that chronic garlic intake significantly decreased lipid peroxidation, while endogenous antioxidants, such as GSH, SOD, and GPx, were found to be increased.Citation57 Supporting this speculation, we have previously shown that AGE administration reversed oxidant responses and improved tissue morphology and function in the stomach, ileum, liver, kidney, and bladder, while the reduced GSH levels were restored in all these tissues.Citation22,58,59 Similarly, renal GSH, SOD, and CAT levels that were decreased in cadmium-intoxicated rats were restored when rats were treated with garlic extract.Citation60
In many inflammatory disorders, important components of the pathological processes are linked to the ability of neutrophils to release several agents that can destroy normal cells and dissolve connective tissue. It has been shown that activated phagocytic neutrophils, when suitably stimulated, secrete enzymes (e.g., MPO, elastase, and proteases) and liberate oxygen radicals,Citation61 and thus further aggravate tissue injury indirectly through activated neutrophils. MPO, an essential enzyme for normal neutrophil function, is released from the neutrophils when they are stimulated by various stimulants.Citation62 In turn, MPO plays a fundamental role in oxidant production by neutrophils. In our observation, elevated MPO levels in the kidney, heart, and lung tissues indicate that neutrophil accumulation contributes to CRF-induced oxidative organ injury. Furthermore, the results also suggest that garlic has a preventive effect through the inhibition of neutrophil infiltration. In accordance with the present results, it was previously shown that the therapeutic action of garlic on different inflammatory events is mediated, in part, by its inhibitory effect on tissue neutrophil infiltration.Citation26,46,63 In accordance with the MPO results, increased pro-inflammatory cytokines TNF-α and IL-1β levels were also suppressed by garlic treatment. Recently, in human umbilical vein endothelial cells, garlic was shown to attenuate TNF-α-induced ROS generation, VCAM-1 expression, NF-κB activation, and adhesiveness for monocytes.Citation64
In conclusion, 3-week garlic supplementation attenuated oxidative injury in both the renal and cardiopulmonary tissues while the renal functions were improved. These observations point to the potential role of garlic supplementation as an adjunct in the therapeutic strategies aimed at retarding chronic kidney disease progression and its cardiopulmonary complications.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Becker BN, Himmelfarb J, Henrich WL, Hakim RM. Reassessing the cardiac risk profile in chronic hemodialysis patients: A hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J Am Soc Nephrol. 1997;8(3):475–486.
- Tepel M, Echelmeyer M, Orie NN, Zidek W. Increased intracellular reactive oxygen species in patients with end-stage renal failure: Effect of hemodialysis. Kidney Int. 2000;58(2):867–872.
- Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161(5):2524–2532.
- Terawaki H, Yoshimura K, Hasegawa T, Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int. 2004;66(5):1988–1993.
- Cottone S, Lorito MC, Riccobene R, Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J Nephrol. 2008;21(2):175–179.
- Maruyama Y, Lindholm B, Stenvinkel P. Inflammation and oxidative stress in ESRD–the role of myeloperoxidase. J Nephrol. 2004;17(Suppl. 8):72–76.
- Siems W, Quast S, Carluccio F, Oxidative stress in chronic renal failure as a cardiovascular risk factor. Clin Nephrol. 2002;58(Suppl. 1):12–19.
- Sener G, Paskaloglu K, Satiroglu H, Alican I, Kaçmaz A, Sakarcan A. L-carnitine ameliorates oxidative damage due to chronic renal failure in rats. J Cardiovasc Pharmacol. 2004;43(5):698–705.
- Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:4–9.
- Vaziri ND. Oxidative stress in uremia: Nature, mechanisms, and potential consequences. Semin Nephrol. 2004;24(5):469–473.
- Rahman K, Billington D. Dietary supplementation with aged garlic extract inhibits ADP-induced platelet aggregation in humans. J Nutr. 2000;130(11):2662–2665.
- Dorant E, van den Brandt PA, Goldbohm R, Goldbohm RA, Hermus RJ, Sturmans F. Garlic and its significance for the prevention of cancer in humans: A critical view. Br J Cancer. 1993;67(3):424–429.
- Kleijnen J, Knipschild P, Terriet G. Garlic, onions and cardiovascular risk factors. A review of the evidence from human experiments with emphasis on commercially available preparations. Br J Clin Pharmacol. 1989;28(5):535–544.
- Ide N, Matsuura H, Itakura Y. Scavenging effect of aged garlic extract and its constituents on active oxygen species. Phytother Res. 1996;10(4):340–341.
- Moriguchi T, Takashina K, Chu P, Saito H, Nishiyama N. Prolongation of life span and improved learning in the senescence accelerated mouse produced by aged garlic extract. Biol Pharm Bull. 1994;17(12):1589–1594.
- Sener G, Sakarcan A, Yegen BC. Role of garlic in the prevention of ischemia-reperfusion injury. Mol Nutr Food Res. 2007;51(11):1345–1352.
- Rietz B, Isensee H, Strobach H, Makdessi S, Jacob R. Cardioprotective actions of wild garlic (Allium ursinum) in ischemia and reperfusion. Mol Cell Biochem. 1993;119(1–2):143–150.
- Numagami Y, Suto S, Ohnishi ST. Attenuation of rat ischemic brain damage by aged garlic extracts: A possible protecting mechanism as antioxidants. Neurochem Int. 1996;29(2):135–143.
- Augusti KT, Sheela CG. Antiperoxide effect of S-allyl cysteine sulfoxide, an insulin secretagogue, in diabetic rats. Experimentia. 1996;52(2):115–119.
- Geng Z, Lau BHS. Aged garlic extract modulates glutathione redox cycle and superoxide dismutase activity in vascular endothelial cells. Phytother Res. 1997;11(1):54–56.
- Wei Z, Lau BHS. Garlic inhibits free radical generation and augments antioxidant enzyme activity in vascular endothelial cells. Nutr Res. 1998;18(1):61–70.
- Kabasakal L, Sehirli O, Cetinel S, Cikler E, Gedik N. Protective effect of aqueous garlic extract against renal ischemia/reperfusion injury in rats. J Med Food. 2005;8(3):319–326.
- Sener G, Sehirli O, Ipci Y, Aqueous garlic extract alleviates ischemia-reperfusion-induced oxidative hepatic injury in rats. J Pharm Pharmacol. 2006;57(1):145–150.
- Batirel FH, Aktan S, Aykut C, Yegen BC, Coskun T. The effect of aqueous garlic extract on the levels of arachidonic acid metabolites (leukotriene C4 and prostaglandin E2) in rat forebrain after ischemia-reperfusion injury. Prostaglandins Leukot Essent Fatty Acids. 1996;54(4):289–292.
- Vaziri ND, Ni Z, Oveisi F, Liang K, Pandian R. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension. 2002;39(1):135–141.
- Gedik N, Kabasakal L, Sehirli O, Long-term administration of aqueous garlic extract (AGE) alleviates liver fibrosis and oxidative damage induced by biliary obstruction in rats. Life Sci. 2005;76(22):2593–2606.
- Talke H, Schubert GE. Enzymatic urea determination in the blood and serum in the Warburg optical test. Wien Klin Wochenschr. 1965;43:174–175.
- Slot C. Plasma creatinine determination: A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17(4):381–387.
- Cuzzocrea S, Reiter RJ. Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur J Pharmacol. 2001;426(1–2):1–10.
- Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Meth. 1990;24(4):285–295.
- Bradley PP, Priebat DA, Christerser RD, Rothstein G. Measurement of cutaneous inflammation. Estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–209.
- Beuge JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310.
- Beutler E. Glutathione in red blood cell metabolism. In: Beutler E, ed. A Manual of Biochemical Methods. New York, NY: Grune & Stratton; 1975:112–114.
- Remuzzi G, Benigni A, Remuzzi A. Mechanism of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116(2):228–296.
- Cho KH, Kim HJ, Rodriguez-Iturbe B, Vaziri ND. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am J Physiol Renal Physiol. 2009;297(1):F106–F113.
- Nakagawa S, Kasuga S, Matsuura H. Prevention of liver damage by aged garlic extract. Phytother Res. 1989;3(2):50–53.
- Kourounakis PN, Rekka EA. Effect of active oxygen species of alliin and allium sativum (garlic) powder. Res Commun Chem Pathol Pharmacol. 1991;74(2):249–252.
- Horie T, Awazu S, Itakura Y, Fuwa T. Identified diallyl polysulfides from an aged garlic extract which protect the membranes from lipid peroxidation. Planta Med. 1992;58:468–469.
- Arora RC, Arora S, Gupta RK. The long-term use of garlic in ischemic heart disease – An appraisal. Atherosclerosis. 1981;40(2):175 –179.
- Kiesewetter H, Jung F, Jung E, Effects of garlic coated tablets in peripheral arterial occlusive disease. Clin Investig. 1993;71(5):383–386.
- Foushee DB, Ruffin J, Banerjee U. Garlic as a natural agent for the treatment of hypertension: A preliminary report. Cytobios. 1982;34(134-35):45–152.
- Ernst E, Weihmayr TH, Matrai A. Garlic and blood lipids. Br Med J. 1985;291(6488):139.
- Horie T, Matsumoto H, Kasagi M, Protective effect of aged garlic extract on the small intestinal damage of rats induced by methotrexate administration. Planta Med. 1999;65(6):545–548.
- Pedraza-Chaverri J, Maldonado PD, Medina-Campos ON, Garlic ameliorates gentamicin nephrotoxicity: Relation to antioxidant enzymes. Free Radic Biol Med. 2000;29(7):602 –611.
- Mukherjee S, Banerjee KS, Maulik M, Dinda AK, Talwar KK, Maulik SK. Protection against acute adriamycin-induced cardiotoxicity by garlic: Role of endogenous antioxidants and inhibition of TNF-alpha expression. BMC Pharmacol. 2003;3:16.
- Omurtag GZ, Guranlioglu FD, Sehirli O, Protective effect of aqueous garlic extract against naphthalene-induced oxidative stress in mice. J Pharm Pharmacol. 2005;57:623 –630.
- Devasagayam TPA, Sundquist AR. Di Mascio P, Kaiser S, Sies H. Activity of thiols as singlet molecular oxygen quenchers. J Photochem Photobiol B. 1991;9(1):105–116.
- Yamasaki T, Li L, Lau BHS. Garlic compounds protect vascular endothelial cells from hydrogen peroxide-induced oxidant injury. Phytother Res. 1994;8(7):408–412.
- Numagami Y, Suto S, Ohnishi ST. Attenuation of rat ischemic brain damage by aged garlic extracts: A possible protecting mechanism as antioxidants. Neurochem Int. 1996;29(2):135–143.
- Savas M, Yeni E, Ciftci H, The antioxidant role of oral administration of garlic oil on renal ischemia-reperfusion injury. Ren Fail. 2010;32(3):362–367.
- Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316–328.
- Sağlam B, Cikler E, Zeybek A, Cetinel S, Sener G, Ercan F. An aqueous garlic extract alleviates water avoidance stress-induced degeneration of the urinary bladder. BJU Int. 2006;98(6):1250–1254.
- Wongmekiat O, Thamprasert K. Investigating the protective effects of aged garlic extract on cyclosporin-induced nephrotoxicity in rats. Fundam Clin Pharmacol. 2005;19(5):555–562.
- Sener G, Sehirli AO, Ipçi Y, Cetinel S, Cikler E, Gedik N. Chronic nicotine toxicity is prevented by aqueous garlic extract. Plant Foods Hum Nutr. 2005;60(2):77–86.
- Ross D. Glutathione, free radicals and chemotherapeutic agents. Mechanisms of free-radical induced toxicity and glutathione-dependent protection. Pharmacol Ther. 1988;37(2):231–249.
- Szabo S, Nagy L, Plebani M. Glutathione, protein sulfhydryls and cysteine proteases in gastric mucosal injury and protection. Clin Chim Acta. 1992;206(1–2):95–105.
- Banerjee SK, Maulik M, Mancahanda SC, Dinda AK, Gupta SK, Maulik SK. Dose- dependent induction of endogenous antioxidants in rat heart by chronic administration of garlic. Life Sci. 2002;70(13):1509–1518.
- Zeybek A, Ercan F, Cetinel S, Cikler E, Saglam B, Sener G. Protective effects of aqueous garlic extract in reducing water avoidance stress-induced degeneration of the stomach, ileum, and liver: Morphological and biochemical study. Dig Dis Sci. 2007;52(11):2984–2992.
- Zeybek A, Cikler E, Sağlam B, Ercan F, Cetinel S, Sener G. Aqueous garlic extract inhibits protamine sulfate-induced bladder damage. Urol Int. 2006;76(2):173–179.
- Suru SM. Onion and garlic extracts lessen cadmium-induced nephrotoxicity in rats. Biometals. 2008;21(6):623–633.
- Sullivan GW, Sarembock IJ, Linden J. The role of inflammation in vascular diseases. J Leukoc Biol. 2000;67(5):591–602.
- Kettle AJ, Winterbourn CC. Myeloperoxidase: A key regulator of neutrophil oxidant production. Redox Rep. 1997;3:3–15.
- Sener G, Satıroglu H, Sehirli AO, Kacmaz A. Protective effect of aqueous garlic extract against oxidative organ damage in a rat model of thermal injury. Life Sci. 2003;73(1):81–89.
- Lee EN, Choi YW, Kim HK, Chloroform extract of aged black garlic attenuates TNF-alpha-induced ROS generation, VCAM-1 expression, NF-kappaB activation and adhesiveness for monocytes in human umbilical vein endothelial cells. Phytother Res. 2011;25(1):92–100.
