Abstract
An assessment of the association of angiotensin-converting enzyme (ACE) gene insertion/deletion (I/D) polymorphism with steroid-resistant nephrotic syndrome (SRNS) risk in children is still controversial. A meta-analysis was performed to evaluate the relation between ACE gene polymorphisms and SRNS susceptibility. The relevant studies were screened from electronic database and eligible investigations were synthesized using meta-analysis methods. Seven investigations were identified for the analysis of association between ACE I/D gene polymorphism and SRNS risk in children, including five in Asians, one in Caucasians, and one in Africans. There was not a markedly positive association between D allele or DD genotype and SRNS susceptibility in Asians (OR = 1.60, p = 0.26; OR = 1.90, p = 0.38) and for Caucasian population (OR = 0.92, p = 0.86; OR = 0.27, p = 0.22). However, an association of D allele with SRNS susceptibility was observed (OR = 4.67, p = 0.003) in Africans, but not for DD genotype (OR = 6.00, p = 0.05). Interestingly, II genotype seemed to play a positive role against SRNS onset for Asians and African children (OR = 0.51, p = 0.02; OR = 0.07, p = 0.02), but not for Caucasians (OR = 0.33, p = 0.30). In conclusion, our results indicate that D allele or DD homozygous might not be a significant genetic molecular marker for the development of SRNS in Asians and Caucasian children. However, D allele seemed be associated with SRNS risk for Africans but DD genotype did not.
INTRODUCTION
Idiopathic nephrotic syndrome (INS) is one of the most common glomerular diseases in children, with a benign prognosis, and most of them satisfactorily respond to steroid therapy.Citation1 Age of initial presentation has an important impact on the disease distribution and response to steroid.Citation2 According to the clinical response to steroids, INS is divided into steroid-sensitive nephrotic syndrome (SSNS) and non-steroid-sensitive nephrotic syndrome (non-SSNS), and non-SSNS is further divided into steroid-dependent nephrotic syndrome (SDNS) and steroid-resistant nephrotic syndrome (SRNS). Most of the children with INS respond well to corticosteroid treatment (SSNS), and about 10% of children with INS are mainly steroid-resistant (SRNS).Citation3 SRNS is at risk of developing end-stage renal disease (ESRD). Some investigations suggest that genetic factors might play an important role in the onset of SRNS.Citation4–6 The angiotensin-converting enzyme (ACE) gene insertion/deletion (I/D) polymorphism, correlating with circulating and cellular ACE concentration,Citation7 has been implicated in the etiology of SRNS and has been investigated in numerous epidemiologic studies. However, the available evidence reported to date is weak, due to sparseness of data or disagreements among the reported investigations. There is rare meta-analysis to explore the association of ACE I/D polymorphism with SRNS susceptibility in children. We performed this meta-analysis to investigate the relation between ACE gene polymorphisms and SRNS risk, with the intention to provide a much more reliable finding on the significance of the association.
MATERIALS AND METHODS
Search Strategy
The relevant studies were searched from the electronic database of PubMed, Cochrane Library, and CBM-disc (China Biological Medicine Database) on 1 September 2010. (steroid resistant nephrotic syndrome OR SRNS) AND (angiotensin converting enzyme OR ACE) were entered into PubMed and Cochrane Library for search, and (idiopathic nephrotic syndrome OR INS) AND (angiotensin converting enzyme OR ACE) AND (gene polymorphisms) were used in CBM-disc. The search in PubMed was limited to humans and English language. We also extended search spectrum to the “related articles” and the bibliographies of all recruited studies. If multiple publications from the same study group occurred, we only recruited the most complete paper into our final analysis.
INCLUSION AND EXCLUSION CRITERIA
Inclusion Criteria
(1) A case–control study; (2) the outcome had to be SRNS; (3) there had to be at least two comparison groups (SRNS group vs. control group); and (4) study conducted in children.
Exclusion Criteria
(1) Review articles and editorials; (2) case reports; (3) preliminary results not on ACE gene or outcome; (4) investigation of the role of ACE inhibitor to disease; (5) investigation of the association of other genes with SRNS or the relation between ACE gene polymorphism and other diseases; (5) association of ACE genotype with INS but not SRNS.
Data Extraction and Synthesis
The information was extracted from each study independently by two investigators as follows: first author’s surname, year of publication, ethnicity of study population, and the number of cases and controls for ACE genotype. Frequencies of alleles were calculated for case group and controls, from the corresponding genotype distribution. The results were compared and disagreements were resolved by discussion.
Statistical Analysis
Cochrane Review Manager Version 5 (Cochrane Library, Oxford, UK) was used to calculate the available data from each investigation. The pooled statistic was counted using the fixed effects model, but a random effects model was performed when the p-value of heterogeneity test was less than 0.1. Results were expressed with odds ratios (OR) for dichotomous data, and 95% confidence intervals (CI) were also calculated. p < 0.05 was required for the pooled OR to be statistically significant. I2 was used to test the heterogeneity between the included studies. A chi-square (χ2) test using a web-based program was applied to determine if genotype distribution of the control population reported conformed to Hardy–Weinberg equilibrium (HWE; p < 0.05 was considered significant), and the study that the genotype distributions in the controls were significantly deviated from HWE was excluded from our sensitive analysis. All descriptive data were expressed as mean ± SD.
RESULTS
Study Characteristics
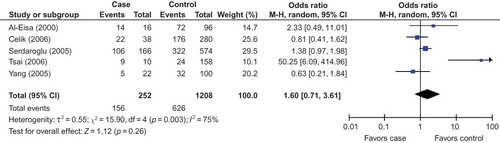
The search yielded 56 references, 40 in PubMed, 1 from Cochrane Library, and 13 in CBM-disc, and 2 investigationsCitation8,9 were identified from the “related articles” and bibliographies of recruited studies. Three studiesCitation10–12 were excluded because they reported the association of ACE genotype with SSNS or non-SSNS but not SRNS. Finally, seven studies were identified for the analysis of the association between ACE I/D polymorphism and SRNS susceptibility in our final review (). Interestingly, all the recruited investigations were performed in children. Five studiesCitation8,13–16 were conducted in Asians, oneCitation3 for Caucasians and oneCitation9 investigation in Africans. Six studies were published in English and oneCitation15 in Chinese. The data of our interest were extracted: first author’s surname, year of publication, ethnicity of study population, and the number of cases and controls for ACE genotype (). These seven investigations contained 151 case series and 823 controls. The average distribution frequency of ACE D allele in Asian patients with SRNS was 64.40%, and the frequency in controls was 48.23%. Furthermore, the average distribution of D allele frequencies in Caucasians was 50.00% in cases and 52.01% for controls. In Africans, the average gene frequency for D allele in SRNS group was 66.67%, and 30.00% for control group. The average distribution frequency of D allele between cases and controls was similar in Caucasians (Caucasians: SRNS/control = 0.96), but not for Asians and Africans (Asians: SRNS/control = 1.34; Africans: SRNS/control = 2.22).
Figure 1. Flow chart for our studies.
Note: ACE, angiotensin-converting enzyme; SRNS, steroid-resistant nephrotic syndrome; CBM, China Biological Medicine; INS, Idiopathic nephrotic syndrome.
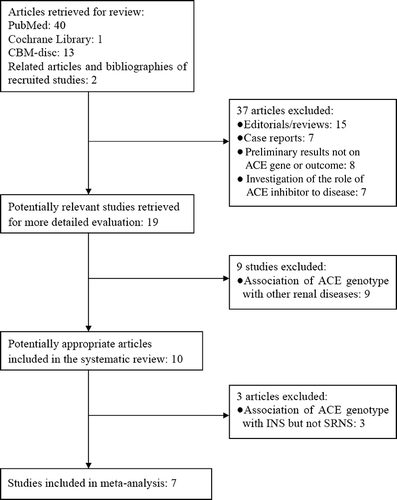
Table 1. Characteristics of the studies evaluating the effects of ACE genes on SRNS risk.
Association of the ACE Polymorphism with SRNS Risk
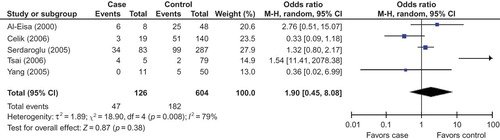
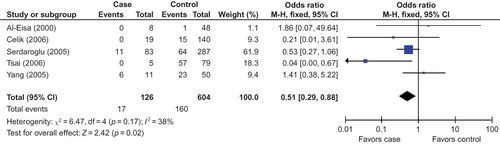
In this meta-analysis, we had not found a significant association between the risk of D allele and SRNS in overall populations (). The random effects OR estimated for the SRNS susceptibility was 1.69 in D allele patients when compared with patients carrying I allele (95% CI: 0.88–3.25; ), with evidence of between-study heterogeneity (p = 0.001; ). Furthermore, DD genotype seemed also not to be associated with SRNS risk in overall people, and the random effects OR estimated for the risk of developing SRNS was 1.72 in DD homozygous patients when compared with both other genes combined (95% CI: 0.53–5.53; ), for the reason that the p-value of heterogeneity test was less 0.0005. Interestingly, we documented that there existed significant association between the II genotype and the risk of SRNS relative to the combination of both other genotypes (OR = 0.43, 95% CI: 0.26–0.71, p = 0.001; ).
Table 2. Meta-analysis of the ACE polymorphisms and SRNS association.
In our study, we found the frequency of D allele in control group of Caucasians or Asians was elevated markedly than that in Africans (Caucasian/Africans = 1.73, Asians/Africans = 1.61). True race-specific genetic effects could affect the results of our analyses for the association of ACE gene with SRNS susceptibility. In order to evaluate the race-specific effect, we divided the population by ethnicity. In Asians, we found the association of D allele or DD homozygous with SRNS risk was not positively significant (p = 0.26 and p = 0.38, respectively; ) ( and ). However, the II genotype seemed to play a protective role against SRNS for Asians (OR = 0.51, 95% CI: 0.29–0.88, p = 0.02; ) (). In Caucasians, there was no significant association between D allele or DD homozygous and SRNS susceptibility (p = 0.86 and p = 0.22, respectively; ). Furthermore, the II genotype might not play a protective role against SRNS onset for Caucasians (OR = 0.33, 95% CI: 0.04–2.68, p = 0.30; ). However, we had found that there existed an association between D allele or II gene and SRNS susceptibility for Africans (D allele: OR = 4.67, 95% CI: 1.69–12.90, p = 0.003; II homozygous: OR = 0.07, 95% CI: 0.01–0.65, p = 0.02; ), but DD genotype was not associated with SRNS risk (OR = 6.00, 95% CI: 1.00–35.91, p = 0.05; ).
Sensitivity Analysis
The gene distribution of control group in the included study was not in HWE, which might be an important reason to cause heterogeneity. In this meta-analysis, sensitivity analysis was performed. The genotype distribution of the control population in oneCitation13 study did not conform to HWE and this investigation was excluded from our analysis. Finally, six studies were recruited into our sensitivity analysis: fourCitation8,14–16 in Asians, one for Caucasian population, and one in Africans. The genotype distribution of the control population in Caucasian children and for Africans conformed to HWE, so the included studies and results for Caucasians and African children were consistent with the previous ones. We only conducted the sensitivity analysis in Asians and for overall populations. The results in our sensitivity analysis were consistent with those in , besides that of the II homozygous for Asians and overall populations (). In our sensitivity analysis, the II genotype seemed not to play a protective role against SRNS in Asians and overall populations (Asians: OR=0.44, 95% CI: 0.07–2.81, p = 0.39; overall populations: OR=0.31, 95% CI: 0.08–1.18, p = 0.09; ).
Table 3. Meta-analysis of the ACE polymorphisms and SRNS association (sensitivity analysis).
DISCUSSION
The genetic origin of renal disease has been a focus of research, especially the recent past. There is significant evidence showing that the renin aldosterone system (RAS) has taken part in the pathogenesis of kidney disease. The level of plasma ACE, constitutively expressed in several types of somatic cells, is linked to an I/D polymorphism of 287 bp in intron 16 of the ACE gene.Citation17 The D allele and DD genotype have been reported to be associated with higher plasma ACE levels.Citation3,7 ACE is a important enzyme of RAS that can convert inactive angiotensin I into a vasoactive and aldosterone-stimulating peptide angiotensin II.Citation18,19 The increased ACE protein expression might be responsible for the elevation of plasma angiotensin II level.Citation20 The increased angiotensin II level makes deleterious actions on renal hemodynamics and induces the protein expression of some other growth factors.Citation21,22 Genetic factors, in particular ACE I/D gene polymorphism, have been suggested to be important in determining the progression of SRNS. SRNS is one of the most common renal diseases in children. Data on the risk factors for the onset of SRNS are insufficient. Furthermore, findings on the association of ACE gene polymorphisms with SRNS susceptibility have been controversial since the first investigation was reported. In order to draw a more convincing conclusion, this meta-analysis was performed to explore the association between ACE I/D gene polymorphism and SRNS risk. In this investigation, seven studies were recruited, five investigations for Asians, one study in Caucasians and one for Africans. In our study, combination of the results in with , we found D allele and DD homozygous were not associated with SRNS susceptibility in Asians and Caucasians. However, D allele was associated with SRNS onset for Africans, and the distribution of DD genotype was favorable to SRNS when compared with that in controls, although there was no statistical significance between them (OR = 6.00 and p = 0.05).
In the included studies for our meta-analysis, Al-Eisa et al.Citation8 had investigated the association of ACE gene I/D polymorphism with clinical characteristics of the INS in Kuwaiti Arab children and found most SRNS patients (75%) had DD genotype. Tsai et al.Citation16 showed that both SSNS and non-SSNS patients in their study had a significantly higher percentage of DD homozygous than the control group; when compared to the SSNS group, a higher incidence of DD genotype was noted among the non-SSNS patients and SDNS patients. Its finding suggested that the DD homozygous might be a risk factor for INS and played a role in the clinical response to steroids. Serdaroglu et al.Citation13 found the D allele was associated with the responses of steroid treatment (including SRNS and SSNS) in Turkey, and the distributions of ACE gene polymorphisms in the SSNS and SRNS groups were similar. Celik et al.Citation14 reported that the distribution of the ACE I/D genotype in the patient group was statistically different from that of the control group, and no statistically significant difference was observed between steroid response and ACE genotypes. We speculated that the frequency of ACE D allele distribution in SRNS of Serdaroglu et al.Citation13 and Celik et al.Citation14 was elevated. However, Yang et al.Citation15 reported that the distributions of ACE gene polymorphisms in the INS and control group were similar, and the ACE gene distributions were not related to the SRNS susceptibility in Chinese children. In our meta-analysis for Asians, an association of DD homozygous or D allele with SRNS risk was not observed, although the D allele frequency in case group was higher than that in controls (1.34-fold elevation). Our results were similar to those of Celik et al.Citation14 In Europe, Sasse et al.Citation3 reported that the distributions of ACE gene polymorphisms in non-SSNS were not significantly different from the normal population and ACE gene polymorphism was irrelevant for steroid responsiveness in Swiss children with INS. Our results for Caucasians were in agreement with those of Sasse et al.Citation3 In Egypt, Fahmy et al.Citation9 found children with INS had a significantly higher percentage of DD genotype or D allele than that in the control group, and found the DD homozygous was associated with the susceptibility of SDNS or SRNS, but not for SSNS. It was similar with our result for Africans.
Ethnicity and age play important roles in the epidemiology of INS.Citation2 In this investigation, the frequency of D allele of control group in Caucasians had a 1.73-fold elevation and that for Asians was about 1.61-fold greater when compared with that in Africans, suggesting a discrepant role of continental differences in gene background and the living environment. It might affect the credibility of the results of association between ACE gene polymorphisms and SRNS risk and the results of overall population were poorly powerful. Furthermore, the factor of age was very important in the epidemiology of SRNS. Last but not least, the genotype distribution of the control population in this study did not conform to HWE which might be a key factor to affect the stability of the conclusion and it was difficult to draw a convincing conclusion. As the results mentioned above, it was necessary to conduct the subgroup analysis and sensitivity analysis in children, and the results would be more credible when compared with the previous ones. So, we included the studies conducted in childhood and divided the population by ethnicity, and sensitivity analysis was conducted to explore whether the results in our study were stable.
Five investigations were recruited into this meta-analysis for Asians. We found that the association of D allele or DD homozygous with the risk of SRNS was not statistically different. When we performed the sensitivity analysis (including four studies) to explore the relation between D allele or DD genotype and SRNS susceptibility, the results were consistent with the previous ones. It might be safe to draw the conclusion that D allele or DD homozygous was not associated with the onset of SRNS in Asians. However, II genotype might play a protective role against SRNS risk in the pooled analysis for five studies, but there was no association between II genotype and SRNS risk when sensitivity analysis was conducted. So, the result for the association of II gene with SRNS susceptibility in Asians was not stable in our meta-analysis.
In Caucasians, no significant association between D allele or DD genotype and SRNS susceptibility was observed and II genotype might not play a protective role against SRNS onset. However, there was only one investigation included in our meta-analysis. At present, reports investigating the relation between ACE gene polymorphism and SRNS risk in Caucasians are rare. Patients with focal segmental glomerulosclerosis (FSGS) respond poorly to steroid therapy and most of them tend to progress to SRNS. Dixit et al.Citation23 investigated the role of ACE gene polymorphisms in the rate of FSGS in Americans and found no association between ACE I/D gene polymorphism and the presence of FSGS, and the distributions of ACE I/D gene polymorphism in FSGS were similar to those in controls. Luther et al.Citation24 reported that the difference in the distribution of D allele in FSGS group in Germany was not statistically significant when compared with controls. We speculated that the ACE I/D gene polymorphism was not associated with the onset of FSGS or SRNS in Caucasian population. More investigations should be conducted in Caucasians to explore the relationship between ACE gene polymorphisms and the risk of SRNS.
In Africans, a significant association between D allele or II gene and SRNS susceptibility was observed. However, the DD genotype was not associated with SRNS onset, but the distribution of DD homozygous in Africans was favorable to SRNS (pooled OR was 6.00 and the p-value was 0.05). In our study, the frequency of D allele in case group had a 2.22-fold elevation when compared with that in controls and the D allele might be a significant risk factor for SRNS susceptibility in African population. However, the number of included study was small (only including one investigation for analysis). So, the conclusions were less powerful in our meta-analysis for African people. In Egypt, Saber-Ayad et al.Citation12 reported the differences of ACE I/D genotype distribution between controls versus the INS patient group, the SSNS group, and the non-SSNS group were not statistically significant, and the ACE I/D genotype distribution in SSNS group was statistically insignificant as compared to the patients in non-SSNS. However, they did not calculate the association of ACE gene polymorphisms with SRNS susceptibility. We performed a test to determine if genotype distribution of the control population reported conformed to HWE in the study of Saber-Ayad et al.,Citation12 and found the p-value was 0.009. So, the results of Saber-Ayad et al.Citation12 might be not stable. Studies on Africans are rare and it is necessary to perform more investigations on the African population which is at high risk for SRNS (frequency of D allele in cases had a 2.22-fold elevation than that in controls) to explore whether there is an association between ACE I/D gene polymorphism and SRNS susceptibility.
In conclusion, we found DD genotype or D allele was not associated with SRNS risk for Asians and Caucasian population, but there was an association between D allele and SRNS susceptibility in Africans. We speculated that the ACE D allele might affect more sensitively SRNS susceptibility in Africans when compared with those in Asian children and Caucasians. The conclusions for Asians were stable in our investigation. However, it was difficult to draw a moderate conclusion for the association between D allele and DD homozygous with SRNS risk in Caucasians and for Africans, for the result that there was only one study recruited for analysis (number of the included study was small). These findings should be regarded cautiously because many other ingredients, such as heterogeneity of enrolled cases, limited statistical power, variable study designs, and different interventions, were closely related to affect the results. Furthermore, whether the I/D gene polymorphism is just linked with other discrete loci involved in the occurrence of SRNS is not clear at the moment. In order to explore whether there exists an exact association between ACE gene polymorphisms and SRNS, further study is required.
LIMITATIONS
Some limitations should be discussed in this meta-analysis. First, heterogeneities may be present, affecting the results of our meta-analysis, although a random effects model has been performed. Second, an important threat to any literature-based review and meta-analysis is that of reporting bias (only including the study reported in English or Chinese). Last but not least, the sample sizes in some studies and the number of included studies are relatively small. Undoubtedly, the limitations mentioned above might affect our final conclusions.
CONCLUSIONS
In conclusion, the results of our study support that DD genotype or D allele was not associated with SRNS susceptibility in Asians and Caucasian children, but D allele was associated with SRNS onset for Africans. The distribution of DD homozygous in Africans was favorable to SRNS; however, the difference was not statistically significant. Furthermore, II genotype seemed to play a positive role against SRNS onset for Asians or African population, but not for Caucasians. However, more case–control association investigations on larger, stratified populations are required to further clarify the role of this ACE I/D gene polymorphism in SRNS susceptibility, especially for Caucasian children and Africans.
ACKNOWLEDGMENT
The authors gratefully acknowledge the most helpful comments on this article received from Professor Liang Rong, Department of Pediatric-Neonatology, Baylor College of Medicine, Houston, TX, USA.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Madani A, Isfahani ST, Rahimzadeh N, Effect of levamisole in steroid-dependent nephrotic syndrome. Iran J Kidney Dis. 2010;4:292–296.
- Chang JW, Tsai HL, Wang HH, Yang LY. Clinicopathological features and prognosis of Chinese children with idiopathic nephrotic syndrome between different age groups. Eur J Pediatr. 2009;168:1189–1194.
- Sasse B, Hailemariam S, Wuthrich RP, Kemper MJ, Neuhaus TJ. Angiotensin converting enzyme gene polymorphisms do not predict the course of idiopathic nephrotic syndrome in Swiss children. Nephrology (Carlton). 2006;11:538–541.
- Buscher AK, Kranz B, Buscher R, Immunosuppression and renal outcome in congenital and pediatric steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2010;5:2075–2084.
- Hildebrandt F. Genetic kidney diseases. Lancet. 2010;375:1287–1295.
- Benoit G, Machuca E, Nevo F, Gribouval O, Lepage D, Antignac C. Analysis of recessive CD2AP and ACTN4 mutations in steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2010;25:445–451.
- Settin A, Elbaz R, Abbas A, Abd-Al-Samad A, Noaman A. Angiotensin-converting enzyme gene insertion/deletion polymorphism in Egyptian patients with myocardial infarction. J Renin Angiotensin Aldosterone Syst. 2009;10:96–100.
- Al-Eisa A, Haider MZ, Srivastva BS. Angiotensin converting enzyme gene insertion/deletion polymorphism in idiopathic nephrotic syndrome in Kuwaiti Arab children. Scand J Urol Nephrol. 2001;35:239–242.
- Fahmy ME, Fattouh AM, Hegazy RA, Essawi ML. ACE gene polymorphism in Egyptian children with idiopathic nephrotic syndrome. Bratisl Lek Listy. 2008;109:298–301.
- Dang XQ, Yi ZW, He XJ, Wu XC, Liu JH, He QN. Angiotensin I-converting enzyme gene polymorphism in children with nephrotic syndrome. Chin J Pediatr. 2000;38:288–291.
- Oktem F, Sirin A, Bilge I, Emre S, Agachan B, Ispir T. ACE I/D gene polymorphism in primary FSGS and steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2004;19:384–389.
- Saber-Ayad M, Sabry S, Abdel-Latif I, Nabil H, El-Azm SA, Abdel-Shafy S. Effect of angiotensin-converting enzyme gene insertion/deletion polymorphism on steroid resistance in Egyptian children with idiopathic nephrotic syndrome. J Renin Angiotensin Aldosterone Syst. 2010;11:111–118.
- Serdaroglu E, Mir S, Berdeli A, Aksu N, Bak M. ACE gene insertion/deletion polymorphism in childhood idiopathic nephrotic syndrome. Pediatr Nephrol. 2005;20:1738–1743.
- Celik US, Noyan A, Bayazit AK, ACE gene polymorphism in Turkish children with nephrotic syndrome. Ren Fail. 2006;28:401–403.
- Yang F, Liu WJ, Liu PP, He GL, Guo ZQ, Liu PL. Study on gene polymorphism of renin angiotensin system in children with nephritic syndrome. J Jinan Univ (Med Ed). 2005;26:246–251.
- Tsai IJ, Yang YH, Lin YH, Wu VC, Tsau YK, Hsieh FJ. Angiotensin-converting enzyme gene polymorphism in children with idiopathic nephrotic syndrome. Am J Nephrol. 2006;26:157–162.
- Bukreeva L, Grigorov A, Kiesewetter H, Hoppe B. Association of angiotensin-converting enzyme intron 16 insertion/deletion polymorphism with history of foetal loss. J Renin Angiotensin Aldosterone Syst. 2009;10:237–240.
- Lambert DW, Clarke NE, Turner AJ. Not just angiotensinases: New roles for the angiotensin-converting enzymes. Cell Mol Life Sci. 2010;67:89–98.
- Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: Recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23:1327–1338.
- Ribeiro-Oliveira A Jr, Nogueira AI, Pereira RM, Boas WW, Dos SR, Simoes ESA. The renin-angiotensin system and diabetes: An update. Vasc Health Risk Manag. 2008;4:787–803.
- Kaneshiro Y, Ichihara A, Sakoda M, Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18:1789–1795.
- Huby AC, Rastaldi MP, Caron K, Smithies O, Dussaule JC, Chatziantoniou C. Restoration of podocyte structure and improvement of chronic renal disease in transgenic mice overexpressing renin. PLoS One. 2009;4:6721.
- Dixit M, Mansur A, Dixit N, Gilman J, Santarina L, Glicklich D. The role of ACE gene polymorphism in rapidity of progression of focal segmental glomerulosclerosis. J Postgrad Med. 2002;48:266–269, discussion 269.
- Luther Y, Bantis C, Ivens K, Fehsel K, Kolb-Bachhofen V, Heering P. Effects of the genetic polymorphisms of the renin-angiotensin system on focal segmental glomerulosclerosis. Kidney Blood Press Res. 2003;26:333–337.