Abstract
Background: Neurohumoral effects have been suggested to affect kidney function. Stroke is a condition where regulation of the renin–angiotensin system and sympathetic nerve activity are altered. Methods: Renal function as estimated by serum creatinine was analyzed over 1 week in 220 patients after acute ischemic stroke. Results: In patients with chronic kidney disease defined as those with serum creatinine >1.2 mg/dL at admission (n = 62), renal function transiently improved, measured by a mean decrease of creatinine of 0.34 mg/dL during the first days after stroke. A significant and transient decrease of creatinine was also observed in patients with diabetes (n = 69) or patients with heart failure (n = 89). In both subgroups creatinine decreased by a mean of 0.49 and 0.24 mg/dL, respectively (p < 0.05 for both). In patients with normal renal function at admission, no change in serum creatinine occurred during the first week after stroke. There was no association between stroke severity and creatinine change. Conclusion: An acute ischemic cerebrovascular event intermittently improves impaired kidney function. The underlying mechanism may involve central regulation of renal function.
INTRODUCTION
The interaction between brain and kidney has been poorly investigated so far. The renin–angiotensin system (RAS) directly and indirectly regulates arterial blood pressure (BP) and also the BP in the brain.Citation1 RAS modulates blood flow; sympathetic nerve activity, which controls vascular tone; and secretion of neurohormones, which influences electrolyte and fluid homeostasis; it also regulates behavioral processes to increase salt and water intake.Citation1 Disturbances of this homeostasis contribute to cardiovascular risk. The renal autonomic nervous system has been identified as a major contributor to the complex pathophysiology of hypertension, states of volume overload (such as heart failure), and progressive renal disease, both experimentally and in humans.Citation2–7 Autonomic dysfunction has been linked to diseases like hypertension or heart disease.Citation8,9 This causes end organ damage to kidney and brain in the form of functional or structural impairment.
Acute stroke impairs multiple brain functions.Citation8 These might be reflected not only by physical disability but also by other regulatory malfunctions. The RAS is generally upregulated in patients with stroke in order to sustain cerebral perfusion.Citation8 This activation is of prognostic importance and is associated with poor prognosis.Citation10 Protein and electrolyte excretion and kidney function have been reported to be disturbed after stroke.Citation11,12
Despite this evidence for a relationship between the central nervous system activity and the kidney, the influence of an acute disruption of central regulation on the kidney function has not been investigated yet. In order to assess potential changes of kidney function after an acute cerebrovascular event, we performed serum creatinine measurements in an observational multicenter cohort study.
METHODS
Patients
This study was performed within a prospective stroke registry maintained by nine neurological departments in Vienna [Vienna Stroke Registry (VSR)]. This study had been approved by the local ethics committees and complied with the Declaration of Helsinki. Details of the VSR have been published elsewhere.Citation13,14 Briefly, between October 1998 and December 2001 all patients with stroke who were admitted to one of the participating centers within 72 h of symptom onset were prospectively documented, on the basis of informed consent given by the patient or the next of kin. Patients were carefully recorded according to a standardized protocol with respect to risk factors, medical history, laboratory and technical investigations, as well as stroke etiology and severity, measured by validated scales. The diagnosis was established clinically and all patients underwent cranial computer tomography or magnetic resonance imaging. Out of a sample of 1440 patients with acute ischemic stroke, the subgroup of subjects with consecutive creatinine measurement during the first week after the ischemic event was filtered; 220 patients had a documented laboratory report and were included in the analysis. The medical history of patients is shown in .
Table 1. Medical history of subjects admitted with ischemic stroke (n = 220).
Statistics
Descriptive data are presented as means ± standard error of the mean (SEM) if not indicated otherwise. The Mann–Whitney U-test was used to check for differences in baseline variables and changes in creatinine levels between groups. For comparison of creatinine courses, analysis of variance (ANOVA) for repeated measurements and Fisher LSD post hoc tests were applied. Skewed variables were log-transformed for ANOVA. Analyses were performed applying the Statistica software package (Release 6; StatSoft, Inc., Tulsa, OK, USA).
RESULTS
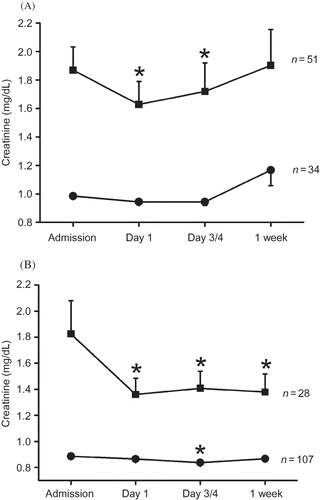
Patients were 73 ± 12 years old and had a mean systolic and diastolic BP of 169 ± 32 and 90 ± 17 mmHg at admission, respectively. Metabolic characteristics are shown in . Serum creatinine values decreased after ischemic stroke by 0.12 ± 0.52 mg/dL and increased again after 1 week in all patients. When patient groups were classified according to renal function at admission, subjects with elevated serum creatinine (>1.2 mg/dL) had a maximum decrease of creatinine (Δ-creatinine) of 0.34 ± 0.92 mg/dL (p < 0.001), while no significant change in serum creatinine was detectable in subjects with normal kidney function (−0.03 ± 0.14 mg/dL Δ-creatinine, p = 0.111; ).
Table 2. Data of subjects with ischemic stroke and normal (≤1.2 mg/dL) or elevated (>1.2 mg/dL) serum creatinine at admission.
Figure 1. Serum creatinine in patients with ischemic stroke and elevated (>1.2 mg/dL, squares, n = 62) or normal creatinine (≤1.2 mg/dL, circles, n = 158) after admission. Data are presented as means ± SEM. Note: *Denotes p < 0.001 versus baseline, ANOVA and Fisher LSD.
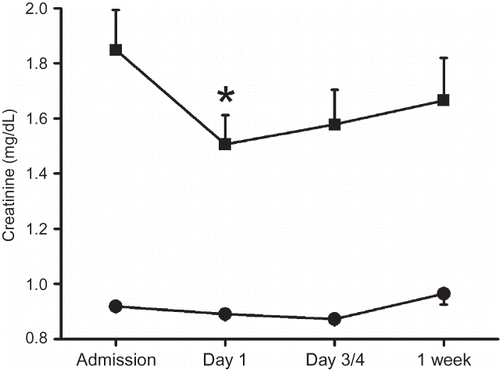
Figure 2. Maximum changes in serum creatinine (Δ-creatinine) after admission for ischemic stroke in patients with elevated (>1.2 mg/dL, gray bars) or normal creatinine (≤1.2 mg/dL, black bars). Obtained from male and female subjects, patients with and without diabetes mellitus (DM), and patients with and without heart failure (HF). Data are presented as means ± SEM.
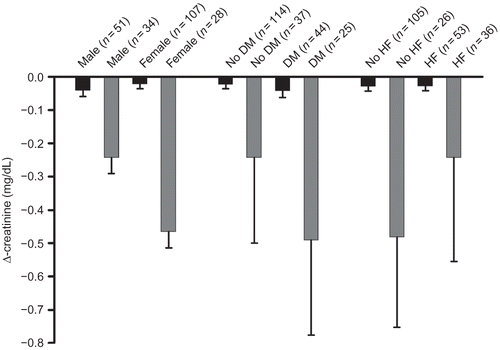
Transient decreases in serum creatinine could also be detected when patients were grouped according to sex or comorbidities (). In patients with ischemic stroke and diabetes or heart failure, serum creatinine decreased significantly on day 1 and on day 3/4, but also in patients with ischemic stroke and without diabetes or heart failure a similar transient change in serum creatinine was observed, though not significant (A and B). Patients with diabetes had higher serum creatinine during the entire observation period than patients without diabetes. Patients with heart failure also had higher serum creatinine than patients without heart failure (mean ± SD 1.28 ± 0.71 mg/dL vs. 1.06 ± 0.48 mg/dL, p = 0.002; A and B). Serum creatinine decreased significantly in men and women with ischemic stroke and elevated creatinine at admission but not in those with normal creatinine at admission ().
No difference in the creatinine course could be detected between patients with or without peripheral vascular disease (n = 34) or coronary heart disease (n = 61) (both p > 0.05; data not shown). Comparable results could be obtained when patients with and without hypertension, ACE-inhibitor therapy, and stroke severity were compared (data not shown). Analysis of hemoglobin and hematocrit courses to exclude potential dilution effects showed no change over time (data not shown).
In multiple group comparison analysis, diabetes or heart failure had no influence on the creatinine course after stroke when the cohort was stratified according to elevated or normal creatinine concentration at baseline (). There was no association between stroke severity scale and the change in creatinine after stroke event ().
Creatinine at hospital admission was associated with sex (p < 0.001), age (p = 0.03), triglycerides (p = 0.02), uric acid (p < 0.001), total protein (p = 0.01), and fibrinogen (p = 0.01).
DISCUSSION
In our study acute ischemic stroke was associated with an improved renal function in patients with elevated creatinine at admission but not in those with normal creatinine. This phenomenon was seen in subgroups of patients with diabetes or heart failure and men or women.
Table 3. Comparison of subgroups to assess the effect of diabetes and heart failure (HF) on the creatinine course stratified according to elevated or normal creatinine (ANOVA and Fisher LSD post hoc test).
Figure 3. Serum creatinine of patients (A) with or without diabetes and (B) with or without heart failure. Data are presented as means ± SEM. Note: *Denotes p < 0.05 versus baseline, ANOVA and Fisher LSD.
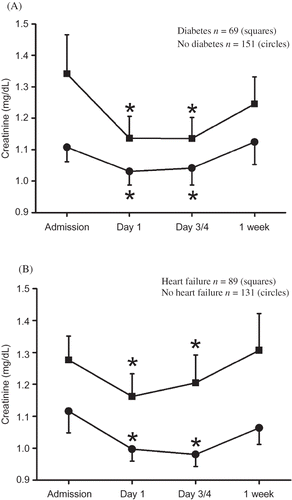
Figure 4. Creatinine course in (A) men and (B) women with ischemic stroke and elevated (>1.2 mg/dL, squares) or normal creatinine (≤1.2 mg/dL, circles). Data are presented as means ± SEM. Note: *Denotes p < 0.01 versus baseline, ANOVA and Fisher LSD.
Brain and kidneys are considered as end organs on parallel trajectories, sharing cardiovascular risk factors, with microvascular pathologic processes mediated by inflammatory and oxidative processes.Citation15,16 Further, brain and kidney have similar low-resistance vascular beds and endothelial structures.Citation15,16 Therefore, vascular dysfunction may lead to defects of the blood–brain barrier in the central nervous system and to impaired glomerular filtration in the kidney.Citation16 Thus, one would speculate a decline of kidney function after cerebral ischemia. Impaired renal function is common among stroke survivors and is associated with an increased risk for subsequent major cardiovascular events, which include acute myocardial infarction, sudden death, and recurrent stroke.Citation17
Patients with diabetes or heart failure under study had higher creatinine concentrations compared with other ischemic stroke patients, which is expected in this group of subjects.Citation18–22 Despite higher creatinine values at admission the decline in creatinine in both groups, a significant decrease in creatinine was also seen in patients without diabetes or heart failure. The highest decrease was detectable in patients with chronic kidney disease (CKD). This was surprising since hyperglycemia, low cardiac output, high BP, or impaired filtration usually deteriorates kidney function in acute situations.Citation23,24 One explanation might be that chronic inflammation in patients with diabetes and heart failure is aggravated by stroke.Citation25–27 Subsequent vasodilatation by local septic conditions in renal arterioles would enhance glomerular filtration and creatinine excretion in the first days after stroke. After normalization of inflammation and vascular tone, renal clearance would return to baseline. Further, it is possible that intensive medical supervision including BP management, hydration, or antithrombotic therapy might positively affect kidney function. On the other hand, this improvement in creatinine was only intermittent and renal function resumed to baseline within 1 week. It is therefore rather likely that the creatinine dynamics are a result of mediators or a mechanism induced by cerebral ischemia. This hypothesis is supported by the finding that comorbidity of diabetes or heart failure did not mitigate the transient improvement in creatinine course. However, there was no relationship between changes in creatinine and stroke severity.
Figure 5. Relationship between individual maximum serum creatinine change and stroke severity scale in 220 patients with ischemic stroke (p = 0.487; r = 0.048, Spearman correlation).
An interesting finding is the fact that creatinine decrease was greater in women than in men. Gender differences are well known for patients with ischemic strokeCitation28 and not related to sex hormones. It is well established that sex differences in stroke patients persist beyond the menopause, and female sex is associated with favorable outcome after brain injury, also in newborn children.Citation28 This study does not provide a ready explanation for the difference observed between the sexes.
The metabolic profile of patients with and without CKD was comparable at admission. Thus, discrepancies, for example, osmotic effects of glucose, can be excluded as an origin for the improvement of kidney function after stroke. In addition, clinical evidence of atherosclerosis as coronary heart disease or peripheral vascular disease did not influence creatinine courses when compared with the general study population. It is therefore unlikely that the presence of potential atherosclerosis affected the results.
In summary, kidney function improved transiently after ischemic stroke and returned to baseline after 1 week in patients with impaired renal function. This effect was also seen in patients with and without heart failure and in those with and without diabetes. This intermittent change in creatinine may be caused by altered neurohumoral regulation of kidney function after stroke.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Cuadra AE, Shan Z, Sumners C, Raizada MK. A current view of brain renin-angiotensin system: Is the (pro)renin receptor the missing link? Pharmacol Ther. 2010;125:27–38.
- DiBona GF. The sympathetic nervous system and hypertension: Recent developments. Hypertension. 2004;43:147–150.
- DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197.
- Wyss JM, Oparil S, Sripairojthikoon W. Neuronal control of the kidney: Contribution to hypertension. Can J Physiol Pharmacol. 1992;70:759–770.
- O’Hagan KP, Thomas GD, Zambraski EJ. Renal denervation decreases blood pressure in DOCA-treated miniature swine with established hypertension. Am J Hypertens. 1990;3:62–64.
- Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45:754–758.
- Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897.
- Sykora M, Diedler J, Turcani P, Hacke W, Steiner T. Baroreflex: A new therapeutic target in human stroke? Stroke. 2009;40:e678–e682.
- Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118.
- Tikhonoff V, Zhang H, Richart T, Staessen JA. Blood pressure as a prognostic factor after acute stroke. Lancet Neurol. 2009;8:938–948.
- Harrigan M. Cerebral salt wasting syndrome: A review. Neurosurgery. 1996;38:152–160.
- Amer M, Haase J, Bie P, Holmegaard SN, Djurberg H. Cerebral salt wasting syndrome and syndrome of inappropriate antidiuretic hormone secretion in subarachnoid hemorrhage caused by ruptured berry aneurysms. Pan Arab J Neurosurg. 2000;4:25–31.
- Lang W, Lalouschek W, for the Vienna Stroke Study Group. The Vienna Stroke Registry—Objectives and methodology. Wien Klin Wochenschr. 2001;113:141–147.
- Lalouschek W, Lang W, Muellner M. Current strategies of secondary prevention after a cerebrovascular event: The Vienna Stroke Registry. Stroke. 2001;32:2860–2866.
- Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv Chronic Kidney Dis. 2008;15:123–132.
- Seliger SL, Longstreth Jr, WT. Lessons about brain vascular disease from another pulsating organ, the kidney. Stroke. 2008;39:5–6.
- Khatri M, Wright CB, Nickolas TL, . Chronic kidney disease is associated with white matter hyperintensity volume. The Northern Manhattan Study (NOMAS). Stroke. 2007;38:3121–3126.
- Yang XL, So WY, Kong AP, . Development and validation of stroke risk equation for Hong Kong Chinese patients with type 2 diabetes: The Hong Kong Diabetes Registry. Diabetes Care. 2007;30:65–70.
- Isomaa B, Almgren P, Tuomi T, . Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689.
- Yang X, So WY, Tong PC, . Development and validation of an all-cause mortality risk score in type 2 diabetes: The Hong Kong Diabetes Registry. Arch Intern Med. 2008;168:451–457.
- Krumholz HM, Chen YT, Vaccarino V, . Correlates and impact on outcomes of worsening renal function in patients > or =65 years of age with heart failure. Am J Cardiol. 2000;85:1110–1113.
- Forman DE, Butler J, Wang Y, . Incidence, predictors at admission and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67.
- Mielniczuk LM, Pfeffer MA, Lewis EF, . Acute decline in renal function, inflammation, and cardiovascular risk after an acute coronary syndrome. Clin J Am Soc Nephrol. 2009;4:1811–1817.
- Melin J, Hellberg O, Fellström B. Hyperglycaemia and renal ischemia-reperfusion injury. Nephrol Dial Transplant. 2003;18:460–462.
- Rizvi AA. Cytokine biomarkers, endothelial inflammation, and atherosclerosis in the metabolic syndrome: Emerging concepts. Am J Med Sci. 2009;338:310–318.
- von Haehling S, Schefold JC, Lainscak M, Doehner W, Anker SD. Inflammatory biomarkers in heart failure revisited: Much more than innocent bystanders. Heart Fail Clin. 2009;5:549–560.
- Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001;14:89–94.
- Liu M, Dziennis S, Hurn PD, Alkayed NJ. Mechanisms of gender-linked ischemic brain injury. Restor Neurol Neurosci. 2009;27:163–179.
