Abstract
Renal microvascular disease reflected directly by peritubular capillary flow reduction and indirectly by renal function impairment has been documented in early diabetic nephropathy (DN) associated with normoalbuminuria and normal serum creatinine concentration. The renal microvascular disease observed in early DN [chronic kidney disease (CKD) stages 1–2] could progress under current practice to late DN (CKD stages 3–5) with a further reduction in peritubular capillary flow. This advanced renal microvascular disease in late DN is characterized by therapeutic resistance to vasodilators and altered vascular homeostasis associated with impaired nitric oxide production. The renal microvascular disease is progressive as the disease severity progresses and eventually induces chronic renal ischemia and a progressive tubulointerstitial fibrosis. Further study has revealed that early DN is associated with an adequately functional vascular homeostasis. Therefore, recognition and treatment of early renal microvascular disease at early DN (stages 1–2) could enhance renal perfusion and restore renal function.
RENAL MICROVASCULAR DISEASE IS UNDERESTIMATED IN RENAL DISEASE IN DIABETES
It has been a general consensus that type 2 diabetes mellitus is recognized as chronic inflammation of vascular disease. It is usually asymptomatically progressive and remains unnoticed until the disease severity is sufficient enough to induce severe ischemia to the vital organ. Nevertheless, a variety of clinical markers have been attempted to identify the vascular damage such as an increased arterial stiffness,Citation1 cardio-ankle vascular index,Citation2 ankle-brachial index,Citation3 peripheral augmentation index,Citation4 vascular calcification,Citation5 and plasma factors such as asymmetric dimethylarginine,Citation6,7 non-high-density lipoprotein cholesterol,Citation8 homocysteine,Citation9 C-reactive protein,Citation10 receptor for advanced glycation end productsCitation11, defective angiogenic factors,Citation12 abnormally elevated antiangiogenic factors,Citation13 von Willebrand factor,Citation14 circulating endothelial cells,Citation15,16 and circulating microparticle.Citation17 All these markers, in fact, reflect macrovascular disease. However, a combination of any of these markers with renal markers such as microalbuminuria,Citation18,19 renal functional impairment, and renal pathology may as well indirectly reflect renal microvascular disease.
With respect to the marker directly reflecting renal microvascular disease, both studies on the structural change in the renal microvasculature and the renal hemodynamics have been the main diagnostic approach serving this purpose.Citation20–27 Unfortunately, the earlier study on histopathology of the kidney attended only the tubulointerstitial structure per se, but rarely mentioned on the renal microvasculature. Intrarenal hemodynamic study has become the first diagnostic instrument that directly sheds light on the renal microvascular disease.Citation28,29 It has come to our surprise that renal microvascular disease has been encountered early in patients associated with steroid-sensitive minimal change idiopathic nephrotic syndrome whose peritubular capillary flow is mildly but definitely defective.Citation24 At this stage of mild renal ischemia, there is no evidence of tubulointerstitial fibrosis. When these nephrotic patients had been followed up long enough, some of them become steroid resistant and the peritubular capillary flow was further reduced down to 30% level of normal. At this level of renal ischemia, the renal histopathology demonstrated not only the peritubular capillary rarefaction reflected by the defective endothelial staining with factor VIII but also the development of tubulointerstitial fibrosis.Citation21 A greater reduction in peritubular capillary flow is associated with a higher magnitude of tubulointerstitial fibrosis. This finding implies that the renal microvascular disease reflected by renal ischemia leads to the development of tubulointerstitial fibrosis. With respect to diabetic nephropathy (DN), renal microvascular disease reflected by an enhanced number of circulating endothelial cells, a reduction in peritubular capillary flow has been documented in early DN associated with normoalbuminuria and normal serum creatinine concentration. Such early DN patients are undifferentiated from the normal population. However, they could be recognized by (1) a defective creatinine clearance, (2) evidence of renal ischemia reflected by peritubular capillary flow reduction, and (3) an abnormally elevated level of fractional excretion of magnesium (FE Mg)—indicating the presence of tubulointerstitial fibrosis, since FE Mg has been previously demonstrated to correlate directly with the magnitude of tubulointerstitial fibrosis.Citation30 Therefore, renal hemodynamics appears to be a useful, noninvasive diagnostic instrument to directly detect all stages of renal microvascular disease. In this regard, another noninvasive diagnostic tool includes Duplex Doppler sonography to detect renal vascular resistanceCitation31 and microalbuminuria.Citation18,19 It has recently been demonstrated that the magnitude of renal microvascular disease predicts the therapeutic responsiveness to vasodilator treatment in chronic kidney disease (CKD). A mild degree of renal microvascular disease is associated with a normal or mildly altered vascular homeostasis with an adequate response to vasodilator treatment.Citation32,33 In contrast, a defective angiogenesis with an impaired nitric oxide production is encountered in the severe degree of renal microvascular disease commonly associated with late-stage CKD (CKD stages 3–5).
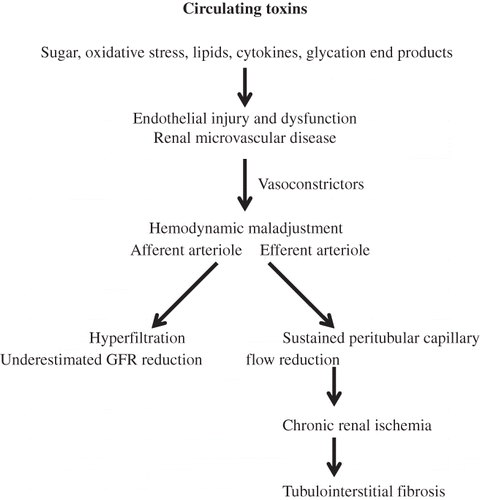
RENAL MICROVASCULAR DISEASE INDUCES RENAL ISCHEMIA AND TUBULOINTERSTITIAL FIBROSIS IN DIABETES
In type 2 diabetes mellitus, numerous toxic substances namely sugar,Citation34 oxidative stress,Citation35–37 cytokines,Citation38 glycation end products,Citation39 lipid,Citation40 altered shear stress,Citation41 and angiotensin IICitation42 have been accumulated and circulated into the renal microcirculation. These circulating toxins continuously play integrated roles and induce injury to the glomerular endothelium as well as the peritubular capillary endothelium. This would induce (1) a detachment of glomerular endothelium from the vascular wall into the vascular lumen so-called circulating endothelial cells andCitation12,15 (2) the remaining endothelium attached to the diseased vascular wall becomes dysfunctioning.Citation28 Collectively, they incriminate in the development of renal microvascular disease. The dysfunctioning glomerular endothelium would upregulate vasoconstrictors, reactive oxygen species, adhesion molecules, and procoagulant surface and alters intrarenal hemodynamics. The altered renal hemodynamics is characterized by a preferential constriction of the efferent arteriole inducing (1) a sustained peritubular capillary flow reduction, a hemodynamic maladjustment phenomenon, and (2) a less degree of constriction of the afferent arteriole, an underestimated glomerular filtration rate reduction (a hyperfiltration phenomenon). The sustained peritubular capillary flow reduction leads to chronic renal ischemia and eventually to the development of tubulointerstitial fibrosis (). In this regard, correction of chronic renal ischemia with vasodilators would be the appropriate therapeutic target to improve renal perfusion and restore renal function. However, it has been rather disappointing to observe the therapeutic resistance to vasodilators in treating CKD patients who have been treated in general at a rather late stage (CKD stages 3–5) due to the insensitive diagnostic markers available such as serum creatinine and microalbuminuria determinations. We have recently demonstrated that such therapeutic resistance to vasodilators is due to altered vascular homeostasis associated with an impaired nitric oxide production.Citation12
RECOGNITION AND TREATMENT OF RENAL MICROVASCULAR DISEASE AT EARLY STAGE (NORMOALBUMINURIA) ARE ESSENTIAL FOR RESTORING RENAL FUNCTION IN DIABETES
Recognition of early renal microvascular disease during the early DN (normoalbuminuria, CKD stages 1 or 2) is essential for therapeutic recovery of renal function. The vascular homeostasis is adequately functional in early DN which implies that there is enough nitric oxide production to respond to vasodilator treatment and dilate the renal microcirculation to correct the renal ischemia and restore renal function. Recognition of early renal microvascular disease could be assisted by (1) a mild degree of renal ischemia, that is, peritubular capillary flow reduction not greater than 30% of normal; unfortunately, the intrarenal hemodynamic study to obtain this information is generally unavailable to detect the underlying renal microvascular disease in most centers; (2) a mild degree of increased renal vascular resistance31;Citation (3) the fact that microalbuminuria is a biomarker for renal microvascular disease with or without macrovascular disease; (4) an increased number of circulating endothelial cells in conjunction with either of the following renal functional impairments such as impaired creatinine clearance and abnormally elevated FE Mg—FE Mg has been demonstrated to correlate directly with tubulointerstitial fibrosisCitation30 and indirectly with the peritubular capillary flow reduction43;Citation and (5) a mildly impaired creatinine clearance (CKD stages 1, 2)—creatinine clearance correlates directly with the renal plasma flow; a reduction in creatinine clearance reflects renal microvascular disease or renal perfusion deficit.
Early recognition of renal microvascular disease during early DN (normoalbuminuria) is essential for therapeutic implementation at early DN under an environment favorable for vascular repair and correction of renal ischemia. Indeed, several pilot therapeutic studies have confirmed that an enhanced peritubular capillary flow and restoration of renal function could be accomplished with vasodilator treatment in normoalbuminuria type 2 DN.Citation44,45 Among the 50 diabetic patients associated with normoalbuminuria, all remain in normoalbuminuria stage and have improved creatinine clearance above the pretreatment values. In contrast, diabetic patients associated with microalbuminuria showed rather fluctuating results of either increasing the magnitude of proteinuria in some or decreasing in the others. The renal function in these groups might show either improvement in creatinine clearance in some patients or a slow progressive decline in creatinine clearance in the others. In diabetic patients with macroproteinuria, they had the tendency to show a progressive decline in creatinine clearance. Therefore, future strategies to prevent renal microvascular disease complications in diabetes are necessary.Citation46
ACKNOWLEDGMENT
This study is supported by the Thailand Research Fund and the National Research Council Fund of Thailand and the Royal Institute of Thailand.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Bellasi A, Furamosca E, Ratti C. Arterial stiffness in chronic kidney disease: The usefulness of a marker of vascular damage. Int J Nephrol. 2011;2011:754832.
- Takenaka T, Hoshi H, Kato N, . Cardio-ankle vascular index to screen cardiovascular disease in patients with end-stage renal disease. J Atheroscler Thromb. 2008;15(6):339–344.
- Paraskevas KI, Kotsikoris I, Koupidis SA, . Ankle-brachial index: A marker of both peripheral arterial disease and systemic atherosclerosis as well as a predictor of vascular events. Angiology. 2010;61:521–523.
- Hefferuan KS, Kuvir JT, Sarnak MK, . Peripheral augmentation index and vascular inflammation in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2011;26(8):2515–2521.
- Bellasi A, Raggi P. Vascular calcification in chronic kidney disease: Usefulness of vascular damage. J Nephrol. 2011;24(Suppl. 18):S11–S15.
- Marin M, Manez S. Pharmacological interventions on asymmetric dimethylarginine, a clinical marker of vascular disease. Curr Med Chem. 2011;18:714–724.
- Anderssohn M, Schwedhelm E, Luneburg N, . Asymmetric dimethylarginine as a mediator of vascular dysfunction and a marker of cardiovascular disease and mortality: An intriguing interaction with diabetes mellitus. Diab Vasc Dis Res. 2010;7:105–118.
- Sniderman A, MoQueen M, Contois J, . Why is non-high-density lipoprotein cholesterol a better marker of the risk of vascular disease than low-density lipoprotein cholesterol? J Clin Lipidol. 2010;4(1):152–155.
- Nilsson K, Gustafson L, Hultberg B. Plasma homocysteine—A marker of vascular disease in elderly patients with mental illness. Clin Biochem. 2010;43(13–14):1056–1059.
- Abdellaoui A, Al-Khaffaf H. C-reactive protein (CRP) as a marker in peripheral vascular disease. Eur J Vasc Endovasc Surg. 2007;34:18–22.
- Kalea AZ, Schmidt AM, Hudson BI. RAGE: A novel biological and genetic marker of vascular disease. Clin Sci. 2009;116:621–637.
- Futrakul N, Futrakul P. Vascular homeostasis and angiogenesis determine therapeutic effectiveness in type 2 diabetes. Int J Vasc Med. 2011;2011:971524, doi:10.1155/2011/971524.
- Futrakul N, Butthep P, Futrakul P. Altered vascular homeostasis in chronic kidney disease. Clin Hemorheol Microcirc. 2008;38:201–207.
- Horvath B, Hegedus D, Szapary L, . Measurement of von Willebrand factor as the marker of endothelial dysfunction in vascular diseases. Exp Clin Cardiol. 2004;9:31–34.
- Del Papa N, Colombo G, Iracchiolla N, . Circulating endothelial cells as a marker of ongoing vascular disease in systemic sclerosis. Arthritis Rheum. 2004;50:1296–1304.
- Futrakul N, Butthep P, Futrakul P, . Glomerular endothelial dysfunction in type 2 diabetes mellitus. Ren Fail. 2006;28:523–524.
- Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: A potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–186.
- Garg JP, Bahris GL. Microalbuminuria: Marker of vascular dysfunction, risk factor for cardiovascular disease. Vasc Med. 2002;7:35–43.
- Futrakul N, Sridama V, Futrakul P. Microalbuminuria—A biomarker for renal microvascular disease. Ren Fail. 2009;31:140–143.
- Lindenmeyer MT, Kaetzler M, Boucherot A, . Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol. 2007;18:1765–1776.
- Futrakul N, Kittikowit W, Yenrudi S. Reduced endothelial factor VIII staining in renal microcirculation correlates with hemodynamic alteration in nephrosis. Ren Fail. 2003;25:757–746.
- Bortoloso E, Dol Prete P, Veotra MB, . Quantitative and qualitative changes in vascular endothelial growth factor gene expression in glomeruli of patients with type 2 diabetes. Eur J Endocrinol. 2004;150:799–807.
- Yenrudi S, Laohapaibul A, Kittikowit W, . A correlation between renal morphology and renal circulation in pediatric nephrotic syndrome. Ren Fail. 2001;23:85–90.
- Futrakul N, Yenrudi S, Sensirivatana R, . Peritubular capillary flow determines tubulointerstitial disease in idiopathic nephrotic syndrome. Ren Fail. 2000;22:329–335.
- Bohle AC, Mackensen-Haen S, Wehrmann M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res. 1996;192:191–195.
- Kang DH, Kanellis J, Hugo C, . Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816.
- Nakagawa T, Kang DH, Ohashi R, . Tubulointerstitial disease: Role of ischemia and microvascular disease. Curr Opin Nephrol Hypertens. 2003;12:233–241.
- Futrakul P, Sitprija V, Yenrudi S, . Glomerular endothelial dysfunction determines disease progression: A hypothesis. Am J Nephrol. 1997;17:133–140.
- Futrakul P, Poshyachinda M, Yenrudi S, . Intrarenal hemodynamic abnormality in severe form of glomerulonephritis: Therapeutic benefit with vasodilator. J Med Assoc Thai. 1992;75:375–385.
- Futrakul P, Yenrudi S, Futrakul N, . Tubular function and tubulointerstitial disease. Am J Kidney Dis. 1999;33:886–891.
- Galesic K, Sabljar-Matovinovic M, Tomic M, . Renal vascular resistance in glomerular diseases—Correlation of resistance index with biopsy findings. Coll Antropol. 2004;28:667–674.
- Futrakul N, Futrakul P. A mildly altered vascular homeostasis in early stage of CKD. Ren Fail. 2009;31:538–543.
- Futrakul N, Butthep P, Chunhakan S, . Vascular homeostasis in early (normoalbuminuric) type 2 diabetic nephropathy. Asian Biomed. 2010;4:987–990.
- Natarajan R, Bai W, Lanting L, . Effects of high glucose on vascular endothelial growth factor expression in vascular smooth muscle cell. Am J Physiol. 1997;273:H2224–H2231.
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028.
- Vesquez-Viver J, Kalyanaraman S, Martasek P, . Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225.
- Shah SV. Role of reactive oxygen metabolites in experimental glomerular disease. Kidney Int. 1989;35:1093–1106.
- Zinman B, Hanley AJ, Harris SB, . Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84:272–278.
- Thomas MC, Tikellis C, Burno WM, . Interactions between renin angiotensin system and advanced glycation in the kidney. J Am Soc Nephrol. 2005;16:2976–2984.
- Berg AH, Scherer PE. Odipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949.
- Malek AM, Izumo I. Molecular aspects of signal transduction of shear stress in the endothelial cell. J Hypertens. 1994;12:989–1000.
- Sachse A, Wolf G. Angiotensin II induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439–2446.
- Futrakul N, Butthep P. Early detection of endothelial injury and dysfunction in conjunction with the correction of hemodynamic maladjustment can effectively restore renal function in type 2 diabetic nephropathy. Clin Hemorheol Microcirc. 2006;34:373–381.
- Futrakul N, Kulaputana O, Futrakul P, . Enhanced peritubular capillary flow and renal function can be accomplished in normoalbuminuric type 2 diabetic nephropathy. Ren Fail. 2011;33(3):312–315.
- Ritt M, Ott C, Raff U, . Renal vascular endothelial function in hypertensive patients with type 2 diabetes. Am J Kidney Dis. 2009;53:281–289.
- Karalliedde J, Gnudi L. Future strategies to prevent renal microvascular disease complications in diabetes. Future Cardiol. 2008;4:77–83.