Abstract
Objective: Matrix metalloproteinases (MMPs) and transforming growth factor beta (TGF-β) were increased in peritoneal dialysis patients with encapsulating peritoneal sclerosis (EPS) and in chlorhexidine gluconate (CG)-induced peritoneal sclerosing animal models. Peroxisome proliferator-activated receptors (PPARs) are the major regulators of key metabolic pathways of various inflammatory responses in fibrosing processes in most tissues. The objective of this study was to investigate the effect of pioglitazone (Pio), a synthetic PPAR-γ ligand, on the development of peritoneal fibrosis in CG-induced EPS rats. Methods: Thirty-two Wistar albino rats were intraperitoneally injected with saline (C group n = 8) or with CG (1.5 mL/100 g; CG group, n = 8). Pio (30 mg/kg/day) was administered orally to another group of CG injected rats (the CG + Pio group, n = 8) and to another control group (Pio group, n = 8) from initiation to the end of this study. After 14 days of Pio administration, the rats were killed and the parietal and visceral peritoneum were harvested. TGF-β, MMP-2, MMP-9, tissue inhibitor of metalloproteinases (TIMP)-1, and TIMP-2 activity assays and a morphological examination of the peritoneal tissues were performed. Results: Pio significantly inhibited thickening of the submesothelial layer, fibrosis, and inflammation in the peritoneum. It also prevented increases in pro-MMP-2, pro-MMP-9, TIMP-1, and TGF-β activities. Conclusion: Pio, via MMP and TGF-β inhibition, may lessen accumulation of peritoneal extracellular matrix and fibrosis to some extent in an EPS model and might be a new approach to the amelioration of EPS.
INTRODUCTION
Peritoneal dialysis (PD) is an established renal replacement therapy modality in end-stage renal disease patients. Although, after long-term treatment, peritoneal fibrosis (PF) results as a serious complication in some patients, causing membrane failure.Citation1 Long-term and progressive fibrosis may lead to encapsulating peritoneal sclerosis (EPS), the irreversible sclerosis of both visceral and peritoneal peritoneum which is associated with the symptoms of ileus and is life threatening.
The peritoneum may be altered with progressive increase of the extracellular matrix (ECM) deposition and neovasculization.Citation2 Peritoneal mesothelial cells (PMCs) secrete ECM and various cytokines, such as interleukin-1b, transforming growth factor beta (TGF-β), and vascular endothelial growth factor.Citation3–5 These cytokines and ECM accumulation are the main factors in the pathogenesis of PF.
Bioincompatible PD solutions and the high glucose and advanced glycation end products (AGEs) in dialysate play a pivotal role in the PF process. Recurrent peritonitis has also been implicated as a cause contributing to PF.Citation6 However, the precise mechanisms leading to ECM accumulation are not fully understood. ECM accumulation is not only one of the characteristic pathological findings in various diseases, but is also the result of an imbalance between the synthesis and the degradation of ECM components, including collagen, fibronectin, and laminin. It is well known that increased production of ECM and decreased production of matrix-degrading proteinases [matrix metalloproteinases (MMPs)] result in the cumulation of ECM leading to fibrosis.Citation7
Thiazolidinediones (TZDs), synthetic peroxisome proliferator-activated receptor (PPAR)-γ ligands, have a central role in insulin sensitization and adipogenesis and are used extensively in patients with diabetes.Citation8 PPARs are involved in the inflammatory cascade; thus, treatment with PPAR agonists reduces inflammation. The actions of PPAR-γ and PPAR-α activation are thought to be due to their ability to downregulate proinflammatory gene expression and inflammatory cell functions, and as such makes them an attractive target for novel drug intervention. PPAR-γ activation by synthetic (troglitazone) or natural (15d-PGJ2) PPAR-γ ligands in mesenchymal cells results in abrogation of TGF-β-induced collagen synthesis.Citation9,10 It has also been demonstrated that TZDs inhibit the expression of various inflammatory proteins like iNOS, TNF-α, and MMP-9 in macrophages.Citation11 There are many experimental studies demonstrating the antifibrotic effect of TZDs. It has been shown that pioglitazone (Pio) reverses the concentric cardiac left ventricular remodeling and fibrosis via MMP-2 inhibition independently in a chronic hypertension model.Citation12 Exposure of human cortical fibroblasts to Pio has caused an antiproliferative effect and reduced ECM production through mechanisms that include reducing MMP-9, tissue inhibitor of metalloproteinases (TIMP)-1, and TIMP-2 activity, independent of TGF-β1.Citation13 In another experimental study, 2 weeks administration of Pio improved hepatic steatosis, prevented liver fibrosis, and reduced preneoplastic lesions in the liver due to the reduced expression of MMP-2, TIMP-1, and TIMP-2 type and procollagen mRNA.Citation14 In a streptozotocin-induced diabetes model, a decrease in MMP-2 expression in the glomeruli of diabetic rats led to impairment of collagen-IV degradation and contributed to the matrix accumulation in diabetic nephropathy. Pio treatment attenuated the decrease of glomerular MMP-2 and the increase of collagen-IV degradation, and thus had curative effects on diabetic nephropathy.Citation15 All these observations have raised questions concerning the role of PPAR-agonists in the prevention of PF via TGF-β and MMP inhibition.
We investigated the effects of Pio on an experimental model of EPS.
METHODS
Animals
A total of 32 female Wistar albino rats (Department of Experimental Animal Sciences, Dokuz Eylul University) weighing 200 ± 20 g were used throughout the experiment. They were kept on a 12 h light dark cycle at 20 CK with 45% humidity in cages with free access to standard rat feed and tap water. Study animals were randomized into four groups.
Group 1 (n:8): the control (C) group, received 0.9% saline
Group 2 (n:8): the Pio group, received Pio and 0.9% saline
Group 3 (n:8): the EPS group, received intraperitoneal chlorhexidine gluconate (CG)
Group 4 (n:8): the treatment group, received CG and Pio (CG + Pio)
Induction of EPS
The rats were injected intraperitoneally with 0.1% CG and 15% ethanol dissolved in saline (3 mL) for 14 days as previously described.Citation16 The control groups (C and Pio) received 0.9 saline in a same amount (3 mL).
Pio (30 mg/kg) was administered daily (group Pio and CG + Pio) by gavage for 14 days.
Samples
Animals were killed following ether anesthesia on day 15. Via a midabdominal incision, parietal and visceral peritoneum (from liver) were removed for histopathological and biochemical examination.
The peritoneum of the rats was dissected and 50% of tissue was spared for zymography and ELISA and the rest was taken for pathological examination.
Pathological Examination
Light microscopic examination and image analysis
Formalin-fixed paraffin-embedded tissue sections from the parietal and visceral peritoneum were stained by hematoxylin and eosin (H&E) and Von Gieson (VG). To measure peritoneal thickness at sections stained with H&E, microscopic images were transferred to a computer through a camera (Olympus BX50, Olympus Optical Co., Tokyo, Japan). Median of thicknesses of visceral and parietal peritoneum at 10 sites was measured using image analysis software.
Likewise, images stained with VG’s stain were also transferred to a computer. Red-stained areas representing collagen were signed by multiple clicking on red-stained regions by the pathologist and similar stained regions were measured by the software (by Mustafa Sakar), and stained area percentages were calculated for each case in 10 areas and the mean value was applied (a,b).
Inflammation was scored semi-quantitatively in H&E-stained sections accordingly—0: no inflammation, 1: mild, 2: moderate, 3: severe.
Vasculopathy was scored as follows, modified from Williams and colleagues—0: normal, 1: subendothelial hyalinization, 2: luminal irregularity and stenosis, 3: luminal obstruction.Citation17,18
Tissue preparation for biochemical analysis
Tissue samples of parietal peritoneum (100 mg) were powdered via liquid nitrogen. Then, they were transferred to 1.5 mL microcentrifuge and a 10-fold higher extraction volume (50 mM Tris–HCl pH 7.4, 1% NP-40, 0.25% deoxycholate, 150 mM NaCl, 1 mM EDTA) was added and homogenized using a sonicator (Sonics&Materials Inc., Danbury, CT, USA). The homogenate was then centrifuged at 10,000 × g for 15 min. The supernatants were used for gelati nase (MMP-2, MMP-9), TIMP-1, and TIMP-2 analyses as described below. All preparation procedures were performed at +4°C. All homogenates were stored at −80°C prior to testing.
Determination of protein content
Protein levels were determined using bicinchoninic acid protein kit (Sigma, Germany). Bovine serum albumin was used as standard.
Gelatin zymography
Both the pro- and the active forms of MMP-2 and MMP-9 were analyzed using gelatin zymography.Citation18 To measure the activities of the MMPs present in the supernatants, gels containing 7.5% polyacrylamide, 0.1% type I gelatin, and 10% SDS were prepared. Equal volumes of homogenate and a nonreducing sample buffer (2×) were mixed and applied to the wells so that each well contained 50 μg protein. Electrophoresis was performed for 4 h, at +4°C, under a constant voltage of 125 V (30 mA/gel). After electrophoresis gels were washed twice with 2.5% Triton X-100 for 15 min to remove SDS, and the gel was subsequently incubated in buffer containing 50 mM Tris–HCl (pH 7.6), 150 mM NaCl, 10 mM CaCl2, 0.5 mM ZnCl2, and 0.02% Brij-35 for 16 h at 37°C. The following day, gels were stained for 1 h with staining solution (0.5% Coomassie brilliant blue, 40% methanol, and 10% acetic acid) and destained in the same solution without Coomassie brilliant blue. MMP marker (Chemi-Con), containing both pro- and active forms of MMP-2 and MMP-9, was used. A clear zone in the blue background indicated the presence of gelatinolytic activity. Computerized densitometry was used to evaluate relative enzymatic activity (UVP BioImaging Systems with a LabWorks 4.6 Image Acquisition Software). The results were given in arbitrary unit per μg protein.
TIMP-2 and TIMP-1 ELISA assay
TIMP-2 and TIMP-1 levels were determined by using an ELISA-based kit (Ray Bio®, no: ELR-TIMP-1-001, ELH-TIMP2-001), according to the manufacturer’s instructions. Duplicate measurements were performed for each sample. The absorbances were measured using an automated ELISA reader (Synergy HT, Biotek Instrument Inc., Winooski, VT, USA). All results were expressed as pg per mg protein.
MMP-2 and MMP-9 activity assays
Total and endogenous MMP-2 and MMP-9 activity in the tissue homogenates were assayed by activity assays (Biotrak Activity Assay Systems, RPN 2631 and RPN 2634, Amersham, Buckinghamshire, UK). Standards and samples were incubated in microplate wells precoated with anti-MMP-2 and -9 antibodies. All reaction steps were performed according to the manufacturer’s instructions. In order to measure the total MMP-2 and -9 content, any bound MMPs in their pro-form were activated using 1 mM p-aminophenylmercuric acetate (APMA). Active MMP-2 and -9 were detected without APMA treatment. The resultant color was read at 405 nm in an automated ELISA reader (Synergy HT, Biotek Instrument Inc.). The concentrations of active MMP-2 and MMP-9 in the tissue homogenates were determined by interpolation from a standard curve. The sensitivities of the assay for MMP-2 and MMP-9 were 0.5 ng/mL. Final tissue values were expressed as pg per mg protein.
TGF-β ELISA
TGF-β level was analyzed by using an ELISA-based kit (R&D Systems® no: RTM-100), according to the manufacturer’s instructions. Duplicate measurements were performed for each sample. The absorbances (at 450 nm) were measured using an automated ELISA reader (Synergy HT, Biotek Instrument Inc.). All results were expressed as pg per mg protein.
Statistical Analysis
The data were expressed as means ± SD. Statistical significance was analyzed using Student’s t-test or Mann–Whitney U-test. A p-value <0.05 was considered significant.
Ethics
The experimental design was approved by the Ethics Committee of Dokuz Eylul University, School of Medicine.
RESULTS
Pathological Results
In the EPS group, all rats developed parietal and visceral peritoneal inflammation and fibrosis. A little vasculopathy was identified in the EPS group; it was not statistically significant. The first finding was that animals of the treatment group (CG + Pio) showed significant reduction in parietal and visceral peritoneal inflammation and submesothelial thickness as well as fibrosis ( and ). The important second finding was that the rats with EPS receiving Pio (CG + Pio group) demonstrated a similar visceral peritoneal inflammation score with the control groups (C and Pio). demonstrates the pathological findings of parietal peritoneum within all groups. demonstrates the pathological findings of visceral peritoneum within all groups. No difference of pathological features was observed between the control and Pio groups ().
Figure 1. Parietal peritoneum—H&E: (A) control, (B) Pio (no difference of pathological features was observed between the control and pioglitazone groups), (C) chlorhexidine gluconate (CG), and (D) CG + Pio [treatment group (CG + Pio) showed significant reduction in parietal peritoneal inflammation and submesothelial thickness].
![Figure 1. Parietal peritoneum—H&E: (A) control, (B) Pio (no difference of pathological features was observed between the control and pioglitazone groups), (C) chlorhexidine gluconate (CG), and (D) CG + Pio [treatment group (CG + Pio) showed significant reduction in parietal peritoneal inflammation and submesothelial thickness].](/cms/asset/d76e976b-3518-4e08-9cc5-91549fb783b6/irnf_a_623498_f0001_b.jpg)
Figure 2. Parietal peritoneum—Von Gieson (selected): (A) control, (B) Pio (no fibrosis was obsereved in the control and pioglitazone groups), and (C) chlorhexidine gluconate (CG), (D) CG + Pio [treatment group (CG + Pio) showed significant reduction in parietal peritoneal fibrosis and submesothelial thickness].
![Figure 2. Parietal peritoneum—Von Gieson (selected): (A) control, (B) Pio (no fibrosis was obsereved in the control and pioglitazone groups), and (C) chlorhexidine gluconate (CG), (D) CG + Pio [treatment group (CG + Pio) showed significant reduction in parietal peritoneal fibrosis and submesothelial thickness].](/cms/asset/9bc1a42b-7213-4043-b9b7-5c2b87c6fd5c/irnf_a_623498_f0002_b.jpg)
Table 1. Pathological results of the parietal peritoneum.
Table 2. Pathological results of the visceral peritoneum.
Gelatin Zymography
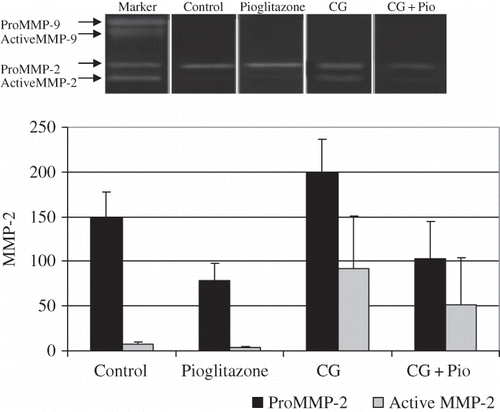
Both the pro- and active forms of MMP-2 and MMP-9 were analyzed by gelatin zymography. MMP-9 levels were under the detection limit for the gelatin zymography. However, it has been demonstrated that pro-MMP-2 was importantly suppressed by Pio treatment in EPS (CG group; p = 0.002). Pio itself induced pro-MMP-2 suppression which was significant when compared with the control group (C; p = 0.001). The expression of active form of MMP-2 was influenced by the EPS model. Both the CG and treatment (CG + Pio) groups had a significant higher MMP-2 activity than the controls. There was little suppression of MMP-2 by Pio which was not significant (p > 0.05). The results are demonstrated together in and .
Table 3. Gelatin zymography results.
MMP-2 and MMP-9 Activity Assays
There was no significant difference in total MMP-2 levels between groups. Endogenous active MMP-2 levels were also similar within the groups (p > 0.05). However, there was an increased pro-MMP-2 level in the CG group, when compared with the controls (C and Pio) (p < 0.05). Pio treatment provided a significant pro-MMP-2 suppression (p < 0.05). Mean pro-MMP-2 level of the treatment group was similar with that of the controls (p > 0.05).
When compared with controls, there was no significant difference in active MMP-9 level in the EPS groups (CG and CG + Pio) (p > 0.05). However, pro-MMP-9 level in the EPS group was higher when compared with that of the controls (C and P) (p < 0.05). Pio treatment provided a significant pro-MMP-9 suppression (p < 0.05). Mean pro-MMP-9 level of the treatment group was similar with that of the controls (p > 0.05) (). There were no significant differences in total MMP-9 levels between groups.
Table 4. Pro-MMP-2, MMP-2, pro-MMP-9, MMP-9 (Activity Assay), TIMP-1, and TIMP-2 (ELISA).
TIMP-2 and TIMP-1 ELISA Assay
Parietal peritoneum TIMP-1 and TIMP-2 levels were also analyzed by ELISA.
When compared with controls (C and Pio), TIMP-1 level was higher in the CG group (p = 0.001). Pio treatment significantly suppressed TIMP-1 (p = 0.009). However, TIMP-1 in the treatment group was still higher than the control groups (C and Pio; p < 0.05).
TIMP-2 levels, however, were similar within the groups (p > 0.05). Although Pio treatment reduced TIMP-2 level, it was not significant (p = 0.07; ).
TGF-β ELISA
The EPS model resulted in an induction of TGF-β level (p = 0.001). Pio treatment provided a significant reduction in TGF-β (p = 0.009). However, TGF-β expression was still higher than the controls (p = 0.003) ().
Table 5. TGF-β activity.
DISCUSSION
Long-term PD treatment results in the loss of ultrafiltration capacity, which can be attributed to an increased effective vascular area. Bioincompatible PD solutions and the high glucose and AGEs in dialysate play a pivotal role in loss of peritoneal membrane integrity.Citation19 Exposure of the peritoneal membrane to high concentration of glucose results in the development of an increased expression of TGF-β and submesothelial collagen and fibronectin accumulation. This results in increased small solute transport but loss of ultrafiltration.
Peritoneal sclerosis is an almost inevitable consequence of PD. In contrast, EPS is a life threatening and usually irreversible condition associated with bowel obstruction, malnutrition, and death. Factors that stimulate cells to promote PF and neoangiogenesis, both inherent in the development of peritoneal sclerosis, include cytokines that are induced by similar factors. Pathological changes in EPS are characterized by submesothelial infiltration of PMN, fibrosis, hyalinizing vasculopathy, and neoangiogenesis.Citation20
PPARs with the ability to downregulate proinflammatory gene expression and inflammatory cell functions have been shown to be expressed in human PMCs (HPMCs).Citation21 Troglitazone has been shown to reduce the expression of TGF-β1 in HPMCs stimulated by 30 mmol/L d-glucose and reduced Fn production. This result was the point of thought that PPAR-γ agonists may have a specific role in ameliorating the course of progressive PF.Citation22
In this study, we intended to cause PF by an EPS model. Pio, a synthetic PPAR-γ agonist, known to suppress inflammation and fibrosis was administered to investigate whether it could affect the activity of MMP-2, MMP-9, and TGF-β or accumulation of peritoneal ECM and fibrosis.
The only study reported recently investigated the effect of rosiglitazone on progression and regression of peritoneal alterations in CG-induced EPS. Compared with the CG rats, 3 weeks of rosiglitazone treatment had beneficial effects on fibrosis but, however, no effect on inflammation and neovascularization. Functional amelioration was also observed by rosiglitazone. However, they did not establish the pathways playing a role in the beneficial effect of rosiglitazone.Citation23
In our study, all rats with CG injection developed parietal and visceral peritoneal inflammation and fibrosis, although little vasculopathy was identified in the CG group.
The most important finding of this study is the decrement of parietal and visceral peritoneal inflammation, submesothelial thickness, and fibrosis in EPS treated by Pio. The inhibition of inflammation may play an important role in the prevention of PF. The interesting finding was that in rats with EPS, Pio treatment provided a similar visceral peritoneal inflammation score with the control groups.
MMPs are increasingly recognized to play an important role in the pathogenesis of fibrosis. They regulate physiologic and pathologic turnover of the ECM and of cell surface proteins.Citation24 These proteolytic enzymes remodel the ECM. MMP activity is regulated by its natural inhibitors, TIMPs. The effect of Pio on MMP-2 and MMP-9 was one of the focus of this investigation. For revealing both forms and levels of MMP-2 and MMP-9, gelatin zymography and activity assay were used together. Gelatin zymography is the electrophoretic technique that allows the discrimination of the pro- (latent) and active forms of MMP-2 and MMP-9 on the same gel according to molecular weight. MMP-9 bands were not detectable on the zymograms. However, total MMP-9 levels were measured by activity assay. This discrepancy may be due to differences in sensitivity and the required sample volume for activity assay and gelatin zymography.
Pro- and active MMP-2 and MMP-9 activities were analyzed by gelatin zymography and ELISA assay. Active MMP-2, pro-MMP-2, and pro-MMP-9 activities were higher in the EPS rats. Pio demonstrated an inhibitory effect on pro-MMP-2 and MMP-9. The correlation between decreased inflammation and fibrosis and suppressed activity of pro-MMP-2, MMP-9 activities reflects the role of gelatinase in the pathophysiological mechanism of PF. With regard to controls, peritoneal TIMP-1 activity was also increased in the EPS group. The inhibitory effect of TIMP-1 on activated MMP-9 reflects the correlation between the activated pro-MMP-9 and upregulated TIMP-1 expression in the EPS groups. Pio treatment provided a significant suppression of TIMP-1 activity. There are several recent papers supporting the data of increased TIMP-1 activity in PF.Citation25
Pio treatment also provided a significant reduction of TGF-β activity, a well-known fibrogenic cytokine which also is demonstrated to play role in PF. It has been demonstrated that PMCs secrete various cytokines including TGF-β.Citation4 In an experimental study, gene transfer of TGF-β1 to the rat peritoneum has resulted in PF.Citation26 TGF-β1-targeting treatments have been investigated in various fibrosis models. In this study, while the EPS rats had the highest TGF-β activity and Pio treatment suppressed it significantly, it was still higher than the controls. The effect of higher doses of Pio on TGF-β expression and whether it would provide further amelioration of fibrosis should be a focus of future investigations.
In summary; Pio-treated mice subjected to CG-induced PF experienced a significantly lower rate in the extent and severity of the histological findings of peritoneal injury. Pio also provided a substantial reduction in the rise of MMP-2 and MMP-9 levels and the expression of TGF-β.
Thus, Pio, via MMP and TGF-β inhibition, may lessen the accumulation of peritoneal ECM and fibrosis to some extent in an EPS model. We propose that this evidence may help to clarify the potential therapeutic actions of Pio in patients with PF.
Declaration of interest: This study was supported by Dokuz Eylul University, Academicals Research Project Supports and the Corporation of Dokuz Eylul Patients and Academicals Research Supports. Pioglitazone was provided by Sanovel Drug Company.
REFERENCES
- Plum J, Hermann S, Fussholler A, . Peritoneal sclerosis in peritoneal dialysis patients related to dialysis settings and peritoneal transport properties. Kidney Int. 2001;59(Suppl. 78):S42–S47.
- Krediet RT. The peritoneal membrane in chronic peritoneal dialysis. Kidney Int. 1999;55:341–356.
- Douvdevani A, Rapoport J, Konforty A, Argov S, Ovnat A, Chaimovitz C. Human peritoneal mesothelial cells synthesize IL-1 alpha and beta. Kidney Int. 1994;46:993–1001.
- Offner FA, Feichtinger H, Stadlmann S, . Transforming growth factor-beta synthesis by human peritoneal mesothelial cells. Induction by interleukin-1. Am J Pathol. 1996;148:1679–1688.
- Mandl-Weber S, Cohen CD, Haslinger B, Kretzler M, Sitter T. Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int. 2002;61:570–578.
- Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev. 1995;5:571–576.
- Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5(8):2145–2154.
- Czoski-Murray C, Warren E, Chilcott J, . Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: A systematic review and economic evaluation. Health Technol Assess. 2004;8:1–91.
- Ghosh AK, Bhattacharyya S, Lakos G, Mori Y, Chen S-J, Varga J. Peroxisome proliferator-activated receptor-γ signaling and profibrotic responses in normal skin disrupts TGF-β fibroblasts. Arthritis Rheum. 2004;50:1305–1318.
- Zheng F, Fornoni A, Elliot SJ, . Upregulation of type I collagen by TGF-β in mesangial cells is blocked by PPARγ activation. Am J Physiol Renal Physiol. 2002;282:F639–F648.
- Li M, Pascual G, Glass CK. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol. 2000;20:4699–4707.
- Shinzato T, Ohya Y, Nakamoto M, Ishida A, Takishita S. Beneficial effects of pioglitazone on left ventricular hypertrophy in genetically hypertensive rats. Hypertens Res. 2007;30(9):863–873.
- Zafiriou S, Stanners SR, Saad S, Polhill TS, Poronnik P, Pollock CA. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol. 2005;16(3):638–645.
- Kawaguchi K, Sakaida I, Tsuchiya M, Omori K, Takami T, Okita K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Biochem Biophys Res Commun. 2004;315(1):187–195.
- Dong FQ, Li H, Cai WM, . Effects of pioglitazone on expressions of matrix metalloproteinases 2 and 9 in kidneys of diabetic rats. Chin Med J (Engl). 2004;117(7):1040–1044.
- Hoff CM. Experimental animal models of encapsulating peritoneal sclerosis. Perit Dial Int. 2005;25(Suppl. 4):S57–S66.
- Sarioglu S, Sis B, Celik A, . Quantitative digital histochemistry with methenamine silver staining in renal allograft biopsies excluding pure chronic allograft nephropathy cases. Transplant Proc. 2006;38(2):490–491.
- Williams JD, Craig KJ, Topley N, . Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–479.
- Heimburger O, Waniewski J, Werynski A, . Periton transport in CAPD patients with permanent loss of ultrafiltration capacity. Kidney Int. 1990;38(39):495–506.
- Io H, Ro Y, Tomino Y, . Morphological changes of peritoneum and expression of VEGF in encapsulated sclerosis rat models. Kidney Int. 2004;65:1927–1936.
- Zhang YF, Yang X, Zhang YJ, . Peroxisome proliferator-activated receptor-gamma is expressed by rat peritoneal mesothelial cells: Its potential role in peritoneal cavity local defense. Am J Nephrol. 2006;26:602–611.
- Yao Q, Ayala ER, Qian JQ, Stenvinkel P, Axelsson J, Lindholm B. Inhibitive effect of troglitazone on TGF-beta(1) and fibronectin expression in human peritoneal mesothelial cells. Clin Nephrol. 2007;68(5):295–301.
- Bozkurt D, Taşkın H, Sezak M, . Rosiglitazone, a peroxisome proliferator-activated receptor agonist, improves peritoneal alterations resulting from an encapsulated peritoneal sclerosis model. Adv Perit Dial. 2008;24:32–38.
- Woessner FJ. MMPs and TIMPs: An historical perspective. Mol Biotechnol. 2002;22(1):33–49.
- Kim JJ, Li JJ, Kim KS, . High glucose decreases collagenase expression and increases TIMP expression in cultured human peritoneal mesothelial cells. Nephrol Dial Transplant. 2008;23:534–541.
- Margetts PJ, Kolb M, Galt T, Hoff CM, Shockley TR, Gauldie J. Gene transfer of transforming growth factor-b1 to the rat peritoneum: Effects on membrane function. J Am Soc Nephrol. 2001;12:2029–2039.