Abstract
Objective: To test the precision and limits of agreement of point-of-care testing (POCT)-based measurement of serum creatinine (Cr) in critically ill patients. Methods: We studied 250 paired blood samples from 82 critically ill patients from a general intensive care unit by simultaneous POCT and central laboratory testing (Jaffé method). Correlation, precision, bias, and limits of agreement were assessed. Possible confounders for interference of noncreatinine chromogens were evaluated by multivariate linear regression analysis. Results: The mean difference in serum Cr measured by central laboratory and POCT was +9.6 μmol/L (95% limits of agreement: −11.2 to +30.4 μmol/L). The mean percentage difference between the two techniques was 8.7% (95% limits of agreement −7.8% to +25.1%). On multivariate regression, the difference in serum Cr was increased with greater hemoglobin and lactate levels but decreased with greater bilirubin, albumin, and calcium levels. Conclusions: Compared with the central laboratory testing, POCT-based measurement of serum Cr in critically ill patients carried a small negative bias. This difference appeared affected by the blood levels of biochemical variables known to affect the Jaffé method. POCT-based Cr measurement appears sufficiently accurate for clinical use.
INTRODUCTION
The provision of biochemical or hematological testing at or near the site of patient care is termed point-of-care testing (POCT).Citation1 The recognition that rapid availability of test results may assist in the more rapid delivery of care has popularized the POCT concept, especially in the emergency department and intensive care unit (ICU). Not only does performance of POCT result in earlier therapeutic action for a significant percentage of patients,Citation2 but economic analysis favors POCT as a more efficient use of healthcare resources.Citation3–5
Although POCT is now an established technology for the measurement and monitoring of arterial blood gases and electrolytes in the ICU, it is not yet established in the assessment of markers of renal function such as serum creatinine (Cr). This is unfortunate because, although serum Cr is an imperfect marker of glomerular filtration rate, it is used in every classification of acute kidney injury (AKI) in the literature.Citation6,7 Moreover, Cr can be used to stratify the severity of AKI and predict mortalityCitation8 and remains the dominant biomarker of AKI in clinical practice. Finally, AKI is common in ICU patients and the limited progress achieved in its treatment so far may, at least in part, be attributed to infrequent monitoring by the central laboratory (once or twice daily). Such infrequent measurements likely contribute to delays in the identification of at-risk patients. These observations argue for the desirability of Cr POCT in ICU in association with the measurement of arterial blood gases. However, for such technology to become widely accepted and applied to patient care, clinicians need to feel confident about its accuracy in critically ill patients.
Accordingly, we sought to evaluate the precision and bias of Cr concentration measured by POCT with an enzymatic amperometric method analyzer (Radiometer ABL837 Flex®, Copenhagen, Denmark) in a cohort of ICU patients. We hypothesized that such POCT would be accurate when compared with central laboratory measurement and show little bias, thus being of sufficient precision for clinical use.
METHODS
The Human Research Ethics Committee of the hospital approved this study and waived the need for informed consent as this study required testing of routinely discarded material and data remained anonymous and confidential.
Morning blood samples from 82 consecutive patients admitted to ICU were simultaneously collected in heparinized blood gas syringes (Rapidlyte; Chiron Diagnostics, East Walpole, MA, USA) and lithium heparin tubes with gel separation (Vacuette; Greiner Labortechnik, Kremsmunster, Austria) from either an intra-arterial or a central venous catheter. The heparinized samples were analyzed immediately by the ICU nurses with the POCT machine while the lithium heparin samples were sent to the central laboratory. Data collected from the POCT machine for analysis were serum Cr (CrP), sodium, potassium, chloride, ionized calcium, lactate, hemoglobin, glucose, blood gases, and pH. Paired blood samples for each dataset simultaneously were sent for analysis to the hospital central laboratory. Laboratory technical staff used a Hitachi multichannel biochemical analyzer (Hitachi 747; Roche Diagnostics, Sydney, New South Wales, Australia) for the measurement of multiple analytes. From the central laboratory, we collected values of serum Cr (CrL), total bilirubin, albumin, total magnesium, phosphate, and urea for evaluation.
The measurement methodology of Cr by POCT using the Radiometer ABL837 Flex® is based on an amperometric technique.
It measures the magnitude of electric flow through two electrodes. This flow is proportional to the concentration of a substance being oxidized or reduced at the electrode side. ABL837 Flex® uses a two-electrode system, one measuring both Cr and creatine and one measuring creatine only. Thus, Cr is the result of the subtraction of the two measures with correction for possible confounders. The electrodes are constituted of a silver cathode and a platinum anode. Cr and creatine molecules are transported across the outer layer of a multilayer membrane. The enzymes creatininase, creatinase, and sarcosine oxidase immobilized between the inner and outer membrane layers change Cr/creatine to glycine, formaldehyde, and H2O2. The H2O2 produced by the enzyme reaction is then transported across the inner membrane to the anode where it affects electrical charge (H2O2 → 2H+ + O2 + 2e–). When a potential is applied to the electrode chain, the oxidation of H2O2 produces an electrical current proportional to the amount of H2O2, which in turn is directly related to the amount of Cr and creatine (or creatine alone). In order to maintain a charge balance between the anode and the cathode, two Ag+ ions need to be reduced for one molecule of H2O2 to be oxidized. To complete the electrical circuit the cathode converts Ag+ in a reduction reaction (where electrons are consumed).
In vitro testing for 61 potential interfering substances at known concentrations has found no detectable sign of interference. Performance of the method has been evaluated for bias against National Institute of Standards and Technology 909b standards.
Statistical Analysis
We used a commercially available program, SPSS for Mac OS X version 18.0.3 (Chicago, IL, USA), for analysis. Data are presented as means and standard deviation or medians and interquartile ranges as appropriate. We first compared the values obtained with the two techniques using the Wilcoxon’s signed rank test. We then constructed a Bland–Altman plotCitation9 to compare the bias and limits of agreement between CrL and CrP. The difference between the two methods, delta Cr (δCr), was defined as the difference between CrL and CrP (CrL – CrP). To study the relationships between δCr and other analytes, we first used Pearson’s correlation coefficient (Pearson’s r). To then assess the independent association between various biochemical analytes and δCr, we performed multivariate linear regression analysis. Factors were excluded from the model if multicollinearity was present at tolerance level of less than 0.4. All statistical comparisons were two sided and a p < 0.05 was considered statistically significant.
RESULTS
We collected 250 paired samples of arterial or central venous blood over a 3-week period from 82 critically ill patients in the ICU. The mean age of study patients was 62 (range 18–87) years and there were 49 males (59.8%). APACHE (Acute Physiology and Chronic Health Evaluation) II score was 18 (±8.7), SAPS (Simplified Acute Physiology Score) II 36.8 (±18.6), length of stay in intensive care (ICU) 4.7 (±6.5), and 15 patients (18%) died in the ICU. Sixty-nine patients (84%) were ventilated on average for 2.1 (±2.1) days and 4 patients were on renal replacement therapy. ICU admission causes included sepsis (11 patients), cardiac surgery (22 patients), acute heart failure and cardiogenic shock (13 patients), and abdominal and other surgeries (25 patients).
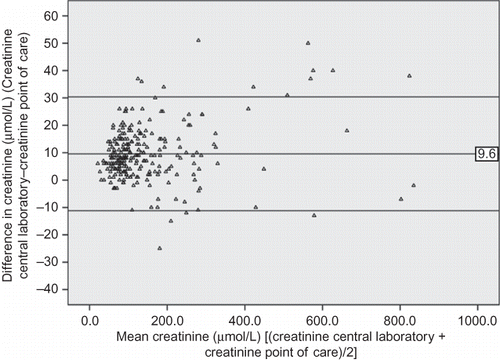
The mean serum Cr as measured by the central lab (CrL) was 154.6 μmol/L compared with the point-of-care (CrP) value of 145 μྒྷmol/L (p < 0.001). The mean difference in serum Cr (δCr) was 9.6 μmol/L, with limits of agreement from −11.2 to 30.4 μmol/L. The mean percentage difference between the two techniques, δCr, was 8.7% (95% limits of agreement −7.8% to 25.1%) of the overall mean serum Cr (149.8 ྒྷmol/L). shows values of other analytes of interest and of blood gases.
Table 1. Analytes of interest and arterial gases values for variables analyzed for interaction with creatinine.
shows the Pearson’s correlation plot for CrL and CrP and shows the Bland–Altman plot of serum Cr measurement. Pearson’s r for CrL/CrP was 0.997 (p < 0.001). Albumin, ionized calcium, and lactate proved at Pearson’s correlation analysis to significantly affect δCr. In particular, albumin and calcium showed a negative relationship (increase in difference in Cr—δCr—at lower values, Pearson’s r −0.188 and −0.249 for albumin and ionized calcium, respectively, p 0.004 and p < 0.001); lactate had a positive relationship (increase in δCr for increasing lactate levels, Pearson’s r 0.238, p < 0.001).
Figure 1. Correlation plot of central laboratory (CrL) versus point-of-care POCT (CrP) creatinine values.
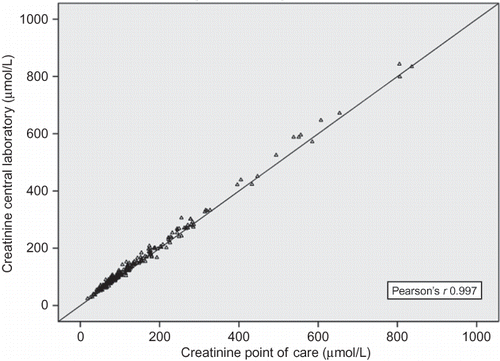
Figure 2. Bland–Altman analysis of creatinine measurement with laboratory (CrL) and point-of-care (POCT; CrP) techniques.
On multivariate regression analysis (see ) δCr was significantly positively associated with serum hemoglobin and lactate and negatively associated with bilirubin, albumin, and calcium. For every 1 g/dL increase in hemoglobin and 1 mmol/L increase in lactate, δCr increased by 1.3 and 1.4 μmol/L, respectively. On the other hand, with every 10 μmol/L decrease in bilirubin, 10 g/L decrease in albumin, and 0.1 mmol/L decrease in calcium, δCr increased by 0.3, 4.5, and 3.0 μmol/L, respectively.
Table 2. Multivariate regression analysis of factors affecting δCr (difference in creatinine calculated as CrL – CrP).
DISCUSSION
Statement of Key Findings
We sought to test the precision and limits of agreement of POCT-based measurement of serum Cr in critically ill patients. We found that POCT estimation of serum Cr by an ICU blood gas machine using an amperometric enzymatic technique yielded values which, on average, were 9.6 μmol/L lower than those obtained through the central laboratory. We also found that the magnitude of the difference between the two techniques varied according to the concentration of some other biochemical variables whose levels change relatively commonly in critically ill patients.
Relationship to Previous Knowledge
Measurement of serum Cr has traditionally been used as a marker of renal function although it is only loosely correlated with glomerular filtration rate.Citation10 Serum Cr measurement in most hospital laboratories relies on the Jaffé (alkaline picrate) method.Citation11 This assay is known to overestimate Cr.Citation11,12 Several chromogens are known to interfere with this method. They include ketones,Citation13 bilirubin,Citation14–17 glucose,Citation18–20 hemoglobin,Citation12,17 and cephalosporin antibiotics.Citation21 Although modifications to the Jaffé method have been made over the years, its specificity for Cr has only moderately improved.Citation22,23 Amperometric enzymatic methods, on the other hand, have been reported not to be affected by chromogens and to yield lower values for serum Cr.Citation24,25 Others, however, found some interaction with chromogens, in particular bilirubin,Citation15 with these methods as well. To overcome its limitations, several different adjustments have been proposed to the Jaffé method.Citation12,16,26 However, standardized calibration is limited leading to variations between laboratories. The National Kidney Disease Education Program acknowledges that although many automated routine methods for measuring Cr meet or exceed the required precision, standardization of calibration does not correct for analytical interferences.Citation27 These observations in their aggregate suggest that central laboratory measurement of serum Cr in critical illness, where ketones,Citation13 bilirubin,Citation14–17 glucose,Citation18–20 and cephalosporin antibioticsCitation21 are commonly high, may be particularly likely to overestimate serum Cr values. They also suggest that enzymatic methods are less likely to lead to such overestimation.
Our primary findings are in accordance with the notion that the Jaffé method overestimates Cr levels, likely because of noncreatinine chromogen interference.Citation12–14,18,21 On the other hand, to our knowledge, the only previous study to assess the precision and bias of Cr measurement by POCT in critically ill patients was performed by Udy et al.Citation28 These investigators found a positive bias of 3 μmol/L between the central laboratory and the POCT measurements. However, this study was retrospective and blood samples for POCT measurement and laboratory measurement were not drawn simultaneously. Our study is prospective and samples were drawn at the same time for POCT and laboratory analysis. A different correction for the Jaffé methodology used in the laboratories of the two institutions could also account for the reported minor difference in bias.Citation28 Importantly, both studies suggest that POCT is accurate and perhaps more accurate than central lab technology.
Our secondary findings that the difference in Cr (calculated as CrL – CrP) was affected by bilirubin, albumin, hemoglobin, calcium, and lactate are consistent with and expand previous knowledge. In particular, this difference was greater with increasing values of hemoglobin and lactate and greater with decreasing values of bilirubin, calcium, and albumin. All these chromogens are known to cause interference with the Jaffé method.Citation12–14,18,21 For example, two studiesCitation15,16 found a lower Cr value with increasing bilirubin concentration that was more evident with the Jaffé method than with an enzymatic assay. A higher Cr value was found with increasing protein concentration with both techniques, while glucose values >30 mmol/L had a positive bias only for the Jaffé method. However, in another study assessing four different enzymatic methods, Schoenmakers et al.Citation24 found that the POCT we assessed had good stability even with high concentrations of bilirubin. Furthermore, Cobbaert et al.Citation17 found greater overestimation of Cr with higher hemoglobin A with the Jaffé method but not with enzymatic methods. They also found that albumin interfered with the Jaffé method but not with the enzymatic methods.
We also found that increasing levels of lactate and decreasing calcium levels were associated with an increase in δCr. Larpent and VergerCitation19 showed that calcium chloride could have an unpredictable effect on Cr measurements with the Jaffé methods but not with an amperometric enzyme technique. Lo et al.Citation20 reported an interaction in dialysate solution between glucose and Cr leading to an increase in creatinine reading. This interaction was enhanced by calcium chloride. A similar finding was also reported by Amici et al.Citation29 who found that this effect of glucose was enhanced by increasing concentrations of calcium lactate. These findings are in agreement with our finding that calcium interferes with Cr readings, but to our knowledge we are the first to report the effect of lactate on increasing Cr measurements.
Glucose has been extensively reportedCitation15,19,20 to increase Cr readings while negligible effects are reported with amperometric enzymatic techniques. We did not find any interference of glucose on δCr in our study. However, most of the studies on glucose interactions with the Jaffé method used very high glucose concentrations (>30 mmol/L).
Significance of Study Findings
Our observations, taken in the context of previous investigations, support the view that POCT of serum Cr is of sufficient accuracy for clinical application and may be more accurate than central laboratory measurements by the Jaffé method. They also suggest that interference by various biochemical analytes is small and unlikely, in most patients, to be of clinical importance. They open the door to frequent monitoring of serum Cr in the ICU with the frequency of monitoring in a given patient being inevitably equivalent to that of arterial blood gases (potentially 4–8 times/day). Whether such automatic increase in monitoring can be translated into changes in treatment and/or outcome now requires investigation.
Strengths and Limitations
Our study has several strengths. It includes prospective design, simultaneous collection of samples, and sufficient statistical power to detect a difference between the two techniques. Limitations included the single-center nature of the study and the lack of measurement of antibiotic concentration (esp. cephalosporins), which is known to affect the value of serum Cr reported by the central laboratory. In addition, we do not know whether some of the substances reported to influence the difference between the central and point-of-care measurements reflect a direct effect or whether they simply act as markers for other unmeasured factors which in turn interfere with measurements (e.g., lactate levels might simply act as markers of ketonemia).
Future Studies
The observations made in this study suggest that POCT for serum Cr is at least equivalent to central laboratory testing. Future studies can now focus on whether POCT can identify more or fewer patients as having AKI according to published criteria and whether such classification proves more accurate at predicting outcome than with central laboratory technology. More importantly, frequent Cr measurement with POCT might allow earlier or better targeted intervention, which in turn might affect outcome. Studies directed at understanding these aspects of POCT are currently underway in our ICU.
CONCLUSIONS
In conclusion, we performed a study to assess the precision, bias, and limits of agreement of serum Cr measurement by a POCT blood gas machine (Radiometer ABL837 Flex®) compared with standard measurement by the central hospital laboratory. We found that POCT had a negative bias of 9.6 μmol/L compared with central laboratory measurement of serum Cr. We also found that the difference in Cr levels measured with the two techniques was affected by lactate, calcium, albumin, and bilirubin levels. However, these interactions were small and more consistent with the known limitations of the central laboratory method than with putative problems with POCT. Further studies assessing the clinical consequences and utility of POCT measurement of serum Cr compared with central laboratory measurement utilizing the Jaffé method appear justified.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Kost GJ. Knowledge optimization theory and application to point-of-care testing. Stud Health Technol Inform. 1999;62:189–190.
- Tsai WW, Nash DB, Seamonds B, Weir GJ. Point-of-care versus central laboratory testing: An economic analysis in an academic medical center. Clin Ther. 1994;16:898–910, discussion 854.
- Kost GJ. Guidelines for point-of-care testing. Improving patient outcomes. Am J Clin Pathol. 1995;104(Suppl. 1):S111–S127.
- Price CP. Medical and economic outcomes of point-of-care testing. Clin Chem Lab Med. 2002;40:246–251.
- Scalise D. Poised for growth. Point-of-care testing. Hosp Health Netw. 2006;80:77–83.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212, doi: 10.1186/cc2872.
- Mehta RLKJ, Shah SV, Molitoris BA, Ronco C, Warnock DG. LAatAKIN. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- Zavada J, Hoste E, Cartin-Ceba R, . A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25:3911–3918.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310.
- Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: Physiological principles. Intensive Care Med. 2004;30:33–37.
- Miller WG, Myers GL, Ashwood ER, . Creatinine measurement: State of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med. 2005;129:297–304.
- Blijenberg BG, Brouwer HJ. The accuracy of creatinine methods based on the Jaffe reaction: A questionable matter. Eur J Clin Chem Clin Biochem. 1994;32:909–913.
- Gerard SK, Khayam-Bashi H. Characterization of creatinine error in ketotic patients. A prospective comparison of alkaline picrate methods with an enzymatic method. Am J Clin Pathol. 1985;84:659–664.
- Daugherty NA, Hammond KB, Osberg IM. Bilirubin interference with the kinetic Jaffe method for serum creatinine. Clin Chem. 1978;24:392–393.
- Srisawasdi P, Chaichanajarernkul U, Teerakanjana N, Vanavanan S, Kroll MH. Exogenous interferences with Jaffe creatinine assays: Addition of sodium dodecyl sulfate to reagent eliminates bilirubin and total protein interference with Jaffe methods. J Clin Lab Anal. 2010;24:123–133.
- Franzini C, Morelli AM, Cattozzo G. Use of a synthetic soluble bilirubin derivative to assess interference in creatinine measurements. Clin Chem. 1991;37:236–238.
- Cobbaert CM, Baadenhuijsen H, Weykamp CW. Prime time for enzymatic creatinine methods in pediatrics. Clin Chem. 2009;55:549–558.
- Da Rin G, Amici G, Virga G, Bardin C, Calzavara P, Bocci C. Correction of glucose concentration interference on Jaffe kinetic creatinine assay in peritoneal dialysis. Am J Nephrol. 1995;15:480–487.
- Larpent L, Verger C. The need for using an enzymatic colorimetric assay in creatinine determination of peritoneal dialysis solutions. Perit Dial Int. 1990;10:89–92.
- Lo SC, Tsai KS. Glucose interference in Jaffe creatinine method: Effect of calcium from peritoneal dialysate. Clin Chem. 1994;40:2326–2327.
- Swain RR, Briggs SL. Positive interference with the Jaffe reaction by cephalosporin antibiotics. Clin Chem. 1977;23:1340–1342.
- Cook JG. Factors influencing the assay of creatinine. Ann Clin Biochem. 1975;12:219–232.
- Haeckel R. Assay of creatinine in serum, with use of fuller’s earth to remove interferents. Clin Chem. 1981;27:179–183.
- Schoenmakers CH, Kuller T, Lindemans J, Blijenberg BG. Automated enzymatic methods for creatinine measurement with special attention to bilirubin interference. Eur J Clin Chem Clin Biochem. 1993;31:861–868.
- Guder WG, Hoffmann GE, Hubbuch A, Poppe WA, Siedel J, Price CP. Multicentre evaluation of an enzymatic method for creatinine determination using a sensitive colour reagent. J Clin Chem Clin Biochem. 1986;24:889–902.
- O‘Leary N, Pembroke A, Duggan PF. A simplified procedure for eliminating the negative interference of bilirubin in the Jaffe reaction for creatinine. Clin Chem. 1992;38:1749–1751.
- Myers GL, Miller WG, Coresh J, . Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18.
- Udy A, O‘Donoghue S, D‘Intini V, Healy H, Lipman J. Point of care measurement of plasma creatinine in critically ill patients with acute kidney injury. Anaesthesia. 2009;64:403–407.
- Amici G, Da Rin G, Bardin C, Gatti PL, Calconi G, Bocci C. Calcium lactate interference in measuring creatinine in peritoneal dialysis fluids by the Jaffe kinetic method. Adv Perit Dial. 1996;12:257–260.
