Abstract
Background: We compared patient characteristics, atherosclerosis, left ventricular hypertrophy, and dialysis practice patterns of peritoneal dialysis (PD) patients with change in cardiovascular disease (CVD) status and no change in CVD status in Chinese PD center. Methods: This study included all patients who started on PD between 1 January 2003 and 30 June 2009 at the Renji Hospital, Shanghai Jiaotong University School of Medicine, China. They were followed up from the date of PD initiation until new-onset CVD. Results: The median follow-up time was 44.13 months. In patients with preexisting CVD, both high triglyceride (1.43 ± 0.89 mmol/L vs. 2.64 ± 1.58 mmol/L, p < 0.001) and the duration of dialysis (45.76 ± 13.28 months vs. 58.68 ± 13.74 months, p < 0.01) were independent predictors of CVD progression and the left atrial dimension, left ventricle septal thickness, left ventricle mass index (LVMI), and intima-media thickness (IMT) also had the difference. On the other hand, in patients without preexisting CVD, the dialysis adequacy and nutritional status are important during the follow-up. Serum albumin in CVD group was lower than in no CVD group (30.86 ± 4.77 g/L vs. 36.04 ± 6.40 g/L, p < 0.05). Creatinine clearance (CCr) and Kt/V in CVD group were lower than in no CVD group (CCr 57.24 ± 13.86 L/week vs. 71.06 ± 23.96 L/week, p < 0.05; Kt/V 1.82 ± 0.41 vs. 2.23 ± 0.57, p < 0.05). Conclusion: In patients with preexisting CVD, it is important to address traditional risk factors such as LVMI, IMT, and lipid profile. In patients without preexisting CVD, we should pay more attention to the nutritional status and PD prescription in order to lower the morbidity of CVD in these PD patients.
INTRODUCTION
Cardiovascular disease (CVD) is a major cause of morbidity and mortality in patients with all forms of chronic kidney disease (CKD).1 A higher prevalence of classic cardiovascular (CV) risk factors (such as smoking, diabetes mellitus, hypertension, and dyslipidemia) and nonmodifiable risk factors (such as age, sex, and family history of CVD) was found in patients with CKD. Dialysis patients have a disproportionately high rate of arteriosclerotic outcome and CVD mortality.Citation2,3 The high prevalence and incidence of CVD in dialysis patients is well described,Citation4 as is the adverse impact of CVD on long-term outcomes.Citation5,6 Numerous studies to date have shown an independent effect of kidney disease itself on CVD outcomes.Citation6–8 The risks of death from cardiac causes and of death from all causes are higher among individuals receiving peritoneal dialysis (PD).Citation9 These results showed that annual CVD mortality rates are much greater in dialysis patients.
We report here the results of data collected from our center for patients with PD. This study attempts to answer additional questions: (1) Is there an independent impact of uremia-associated risk factors on the change of CVD status in PD patients? (2) What risk factors should guide us to prevent or retard progression of CVD in those who have had previous CVD events and those who have no preexisting CVD events?
MATERIALS AND METHODS
Patient Selection
In this study, 254 patients who started on PD between 1 January 2003 and 30 June 2009 at Shanghai Renji Hospital, Shanghai Jiaotong University School of Medicine, China, were considered eligible and were followed up at a median of 44.13 months (range 6–84 months) until 31 December 2009. All patients were Chinese. All patients were regularly monitored at our center and had started their PD treatment at least 6 months before enrollment. Exclusion criteria were survival <6 months, transfer to hemodialysis or kidney transplantation within 6 months, having an active systemic inflammatory disease, and/or having unstable organ disease, such as hepatic failure.
Baseline data were collected regarding patient’s demographic characteristics, laboratory values, PD prescription, and duration on dialysis before enrollment. All of the included patients were prescribed a glucose-based PD solution with at least three 2 L exchanges per day. The total number of episodes of peritonitis and date of every episode of peritonitis were collected. Peritonitis rates were recorded by dividing the months of PD at risk by the number of episodes. All deaths, peritonitis episodes, and other patient outcomes were carefully tracked and recorded. We recorded data of comorbid diseases such as diabetes mellitus and the presence of traditional major CV risk factors [(hypertension, dyslipidemia, body mass index (BMI)] and CVD at the time of PD initiation. BMI was calculated as BMI = weight (kg)/height (m2). After the patient had been recumbent for at least 15 min, brachial arterial blood pressure was measured by using a standard mercury sphygmomanometer.
Informed consent was obtained from each participant, and the ethics board institution approved the study.
Data Collection
Dialysis adequacy data, namely indices of weekly Kt/Vurea and weekly creatinine clearance (CCr), for both peritoneal function and residual renal function were documented using PD-ADEQUEST (Baxter Healthcare Corporation, Chicago, IL, USA). Peritoneal transport characteristics were measured by the dialysate-to-plasma creatinine ratio (D/P Cr) at 4 h in a standard peritoneal equilibration test. Nutritional status was assessed by normalized protein catabolic rate (nPCR) from 24 h dialysate and urine collections. Biochemical tests including blood urea nitrogen, serum creatinine, glucose, calcium and phosphorus levels, serum albumin, and serum C-reactive protein were also examined using standard equipment (ADVIA1650, Bayer Corporation, Tarrytown, NY, USA).
Definitions of Cardiovascular Disease
CVD was defined by history of CV event or condition and/or current symptoms according to the New York Heart Association (NYHA) and the Canadian Cardiovascular Society (CCS) classifications for heart failure and angina, respectively.Citation10,11 CV event history included history of myocardial infarction, angina, coronary artery bypass graft or angioplasty, transient ischemic attack or cardiovascular accident, peripheral vascular disease, and congestive heart failure. Research assistants administered standardized questionnaires that describe symptoms associated with each class of heart failure or angina to each patient, and symptoms were graded by using conventional NYHA and CCS classifications.
Change in CV status was defined as either change in NYHA or CCS classification or a cardiac-related hospitalization.Citation12
Carotid Ultrasound
Carotid B-mode ultrasound was performed at baseline by an independent investigator using a 10 MHz linear array transducer (HP-HX, Georgia, USA). Intima-media thickness (IMT) was evaluated as the distance between the luminal–intimal interface and the medial–adventitial interface. The far wall of the common carotid artery, approximately 5–10 mm proximal to the carotid bulb, was used for the measurements of IMT on both sides. An atherosclerotic plaque was defined as a localized intima-media thickening of >1 mm and at least a 100% increase in thickness compared with adjacent wall segments.
Echocardiography
Imaging and Doppler echocardiography was performed in all of the participants in this study. Studies were performed with echocardiography M-mode echo, using a Hewlett-Packard Sonos 1000 with a 2.5 MHz transducer (Hewlett-Packard, Georgia, USA). All of the measurements were performed by a trained investigator who was blind to the clinical data of the subjects. Measurements included left atrial dimension (LAD), left ventricle septal thickness (LVST), left ventricle posterior wall thickness, left ventricle diameter at end-diastole (LVDd), and left ventricle diameter at end-systole (LVDs). All data were obtained using the American Society of Echocardiography recommendations.Citation13,14
LV mass was considered an unadjusted variable and was normalized by body surface area and expressed as left ventricle mass index (LVMI).Citation15 Left ventricular ejection fraction (LVEF) was calculated as 100 × (LVDd – LVDs)/LVDd.
Outcomes of Interest
The major outcomes of interest for this analysis included new-onset CVD and change in CVD status.
Statistical Analysis
To analyze the data, patients were divided into two groups according to whether they had CVD or not. Data were described as means ± SDs for those with normal distribution and as medians and interquartile ranges for asymmetrical distribution. Differences in the patients’ demographic, clinical, and laboratory parameters between the two groups were evaluated by a t-test. A comparison of the percentages of the two groups was made with a chi-square test (χ2).
Table 1. Patient characteristics associated with CVD status in patients with and without CVD.
Table 2. Patient echocardiography and sonographic characterization associated with CVD status in patients with and without CVD.
Univariate odds ratios were adjusted by using logistic regression. The logistic regression was performed to determine the association between patient characteristics and presence of CVD and then to predict change in CVD status. Survival curves were generated by the Kaplan–Meier method and compared by the log-rank test. Statistical analysis was performed using SPSS for Windows software, version 13.0 (SPSS Inc., Chicago, IL, USA). A two-tailed p-value <0.05 was considered statistically significant.
Table 3. Conventional and associated risk factors in patients with and without CVD.
Table 4. Echocardiography and sonographic characterization in patients with and without CVD.
RESULTS
Baseline Characteristics
In the prospective study, 254 patients (109 men and 145 women) participated. shows the baseline demographics of the whole cohort. The mean calculated CCr was 67.85 ± 21.39 L/week/1.73 m2 and Kt/Vurea was 2.17 ± 0.54. The prevalence of the causes of kidney disease paralleled those in the national registries: glomerulonephritis 61 (24%), diabetes 48 (18.9%), hypertension 30 (11.8%), polycystic kidney disease 8 (3.1%), obstructive nephropathy 8 (3.1%), pyelonephritis 6 (2.4%), lupus nephritis 4 (1.6%), vasculitis 4 (1.6%), and others/unknown 85 (33.5%).
Change in CVD Status during Follow-Up
Of the 254 patients included in this analysis, 23 patients died; 15 (15/23, 62.5%) patients died with CVD. The median duration of follow-ups was 44.13 months. During the follow-up period, 93 of 254 (93/254, 36.6%) patients developed new or worsening cardiac symptoms or were hospitalized for cardiac disease. Of the 144 patients who had no preexisting CVD, 53 (36%) developed new CVD, this in contrast to the 110 patients with preexisting cardiac disease, of which 40 (36%) developed a new event or worsening of CVD.
and describe the results of a univariate analysis comparing the association of conventional and uremia-related variables with change in CVD status in patients with and without preexisting CVD. Note that diabetes at baseline predicts changes in CVD status in both groups of patients.
In patients who had no preexisting CVD, compared with the patients who developed CVD in the follow-up period, no CVD patients have higher CCr, Kt/Vurea, and nPCR. Lower albumin is also associated with changes in CVD status. There were no apparent associations of uremia-specific factors such as hemoglobin, calcium phosphate product, and intact parathyroid hormone (iPTH) levels with change in CVD status.
However, in the patients who had preexisting CVD, higher serum triglyceride levels and longer dialysis durations predicted worsening of CVD over the follow-up period (). shows that patients with preexisting CVD have larger LAD, LVST, and LVMI. There was a clear association between carotid atherosclerosis and CVD events. Patients with preexisting CVD events have higher carotid IMT and more carotid plaques. It shows that LAD, LVST, LVMI, IMT, and carotid plaques were associated with the change of CVD status in this group.
Prevalence of Cardiovascular Disease: A Comparison of Conventional and Uremia-Related Risk Factors
Comparisons between patients with and without CVD at the follow-up period are presented in . The comparison of adequacy data showed that, compared with patients without CVD, CVD patients had lower total Kt/Vurea and total CCr, whereas there was no difference in nPCR and D/P Cr. The total number of episodes of peritonitis was recorded, and peritonitis was most frequently found in CVD patients. Peritonitis was the most frequent in CVD group. Those with CVD at this period were likely to be diabetic and have longer dialysis duration, higher triglycerides, lower albumin, and lower prealbumin.
shows that patients with CVD have larger LAD, LVST, and LVMI than those without CVD. Patients with CVD events have higher IMT.
The multiple linear regression models in show that higher triglycerides, lower albumin, and longer dialysis duration were independent predictors of CVD events. Again, peritonitis and IMT were factors associated with the increased probability of CVD presence during the follow-up period.
Table 5. Multivariate analysis of risk factors associated with the presence of CVD.
Patient Survival and Predictors of Mortality
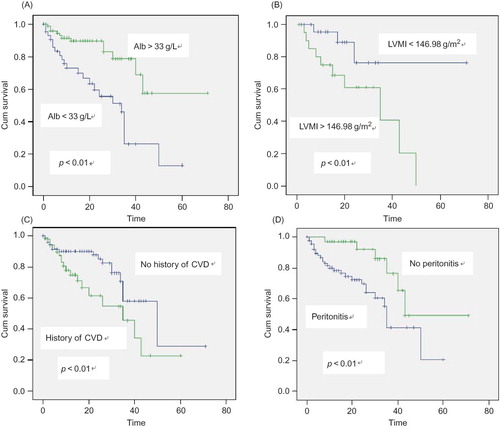
Kaplan–Meier analysis of albumin is seen in Figure 1A, with an observed decrease in patient survival seen with albumin levels of <33 g/L when compared with >33 g/L. We saw a marked difference when we divided patients into those with high and those with low LVMI levels (). The survival curves for patients in show the impact that CVD has at baseline on the survival rate. The prevalence of peritonitis is also associated with CVD events (). Peritonitis and the presence of CVD, which appear to be independent risk factors of mortality, frequently coincide.
DISCUSSION
Cardiac disease is the single most important cause of death among patients receiving long-term dialysis, accounting for 44% of overall mortality.Citation3,9 Both traditional and nontraditional CV risk factors are very prevalent in the incidence of CVD events in this population. We outlined not only the importance of traditional risk factors such as diabetes and dyslipidemic changes but also the nontraditional risk factors such as uremia-related toxin, malnutrition, inflammation, and anemia in this population.
This study further shows the association of conventional risk factors with kidney function decline and also shows an additional association of CVD presence with kidney survival. We have demonstrated many uremia-related risk factors associated with CVD events, CCr, Kt/Vurea, and the duration of PD. Our studies suggest that the degree of uremia or reduced total urea and CCr may be responsible for the progressive CVD observed in long-term continuous ambulatory peritoneal dialysis (CAPD) patients, although the exact uremic toxin causing CVD needs further determination. If the patients who did not have preexisting CVD developed CVD during the follow-up, this population has lower CCr, Kt/V, and nPCR. Patients who have had PD for a longer duration and who have lower CCr, Kt/V, and nPCR are more likely to have experienced CVD events. It is important to caution that our study is a single-center study in nature. Furthermore, multicenter prospective study is therefore needed to determine if there is indeed a cause and effect relationship between uremic toxin and CVD.
In the study presented here, low serum albumin was a strong predictor of mortality in the univariate analysis, which is in agreement with several other reports. Previous studies showed hypoalbuminemia was associated with progressive left ventricular hypertrophy (LVH) and cardiac failure in dialysis patients.Citation16 Foley et al.Citation17 reported that hypoalbuminemia and hyperalbuminemia were associated with the development of de novo and recurrent cardiac failure and ischemic cardiac disease in hemodialysis (HD) patients and in patients on CAPD. This also suggests that malnutrition and CVD are interrelated, although serum albumin is far from an ideal indicator of nutritional status. Especially compared with patients who had preexisting CVD, those who had no preexisting CVD are more hypoalbuminemic if they developed CVD during the follow-up time. This suggests that the novel association between PD and severity of CVD may be mediated in part by hypoalbuminemia. Serum albumin was also a strong predictor of mortality: if serum albumin is lower than 33 g/L, the risk of CVD is higher.
We have a measure of LAD, LVST, and LVMI. LVMI as an indicator of LVH has recently been described as predicting morbidity and mortality and contributing to cardiac dysfunctionCitation18 in cardiac populations. Our analysis shows that LVMI is significantly different between those patients with and without CVD at follow-up time. In our PD patients, ischemic heart disease group had higher LVMI and LVST. Of note, we also found that LVMI predicts incident CVD events. This finding indicates that the study of the left atrium may provide not only important anatomic details that are useful in interpretation of cardiomyopathy in CAPD patients but also complementary prognostic information for risk stratification in this condition.
Atherosclerosis is a manifestation of the pathophysiology underlying CVD. The links between carotid IMT and atherosclerosis are well established and IMT measurements, as markers of atherosclerosis, have contributed greatly to the understanding of atherosclerosis progression.Citation19 As well as carotid IMT, carotid plaques are also a measure of atherosclerosis, perhaps having different attributes or risk associations but still closely related.Citation20 In our study, if patients had a CVD history before dialysis, the carotid plaques and IMT measurements predicted a risk for CVD during the follow-up period. The IMT and plaque measurements are all sensitive markers for arteriosclerosis after the patients accepted PD treatment.
Anemia is also a complication in patients with CKD and those with renal insufficiencies. Observational studies have shown that anemia is a risk factor for adverse CVD outcomes in dialysis patients. In a 1996 study, Foley et al.Citation21 showed that a low Hb level (<8.8 g/dL) was a risk factor for all-cause mortality in univariate analysis. In our study, the therapeutic potential of recombinant erythropoietin (EPO) in treating patients with anemia and PD made correction of anemia a reality. With the average hemoglobin level being around 100.4 g/dL, we did not find an association between Hb and CVD. Maybe the patients we observed got their desirable Hb in chronic PD period.
Nevertheless, a number of weaknesses are inherent in this study design, including biases such as patient selection and survival. Our study is a single-center population study; only one center with a limited number of patients was included, so it may be difficult to extrapolate the results to all PD patients. Some prospective longitudinal studies are needed to evaluate the risk factors of CVD in PD patients.
In summary, on the basis of our findings, the prevalence of CVD in the population with PD is high. We feel that greater efforts must be adopted to increase the awareness of the physician’s involvement with PD patients with regard to CVD. This could be done by considering adequacy of dialysis, nutritional status, LVH, carotid atherosclerosis, and past history of CVD—all in order to improve the CV outcome of such a high-risk population.
ACKNOWLEDGMENTS
We would like to thank the doctors of the Renal Division, Renji Hospital Shanghai Jiaotong University School of Medicine, for their work. This work was supported in part by grant [2008] Nos. 49 and 09dZ1973600 from the Shanghai PuDong Society Development Burea, Science and Technology Commission of Shanghai Municipality China. The project was also sponsored by SRF for ROCS, SEM.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Levey AS, Beto JA, Coronado BE, . Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32:853–906.
- Weiner DE, Tighiouart H, Stark PC, . Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis. 2004;44:198–206.
- Mark JS. Cardiovascular complications in chronic kidney disease. Am J Kidney Dis. 2004;41(S5):S11–S17.
- Parfrey PS. Cardiac disease in dialysis patients: Diagnosis, burden of disease, prognosis, risk factors and management. Nephrol Dial Transplant. 2000;15:58–68.
- Chertow GM, Normand S-LT, Silva LR, McNeil BJ. Survival after acute myocardial infarction in patients with end-stage renal disease: Results from the cooperative cardiovascular project. Am J Kidney Dis. 2000;35:1044–1051.
- Herzog CA. Poor long-term survival of dialysis patients after acute myocardial infarction: Bad treatment or bad disease? Am J Kidney Dis. 2000;35:1217–1220.
- Hemmelgarn BR, Ghali WA, Quan H, . Poor long-term survival after coronary angiography in patients with renal insufficiency. Am J Kidney Dis. 2001;37:64–72.
- Beattie JN, Soman SS, Sandberg KR, Yee J, Borzak S, McCullough PA. Determinants of mortality after myocardial infarction in patients with advanced renal dysfunction. Am J Kidney Dis. 2001;37:1191–1200.
- Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339:799–805.
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(Suppl. 3):112–119.
- Mallamaci F, Zoccali C, Tripepi G, . CREED investigators: The cardiovascular risk extended evaluation. Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int. 2001;59:1559–1566.
- Adeera L, Ognjenka K, Brendan B, . Cardiovascular disease in patients with chronic kidney disease: Getting to the heart of the matter. Am J Kidney Dis. 2001;38:1398–1407.
- Schiller NB, Shah PM, Crawford M, . Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. Am Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367.
- Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 1978;58:1072–1083.
- Devereux RB, Alonso DR, Lutas EM, . Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–458.
- Moon KH, Song IS, Yang WS, . Hypoalbuminemia as a risk factor for progressive left ventricular hypertrophy in hemodialysis patients. Am J Nephrol. 2000;20:396–401.
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol. 1996;7:728–736.
- Harty J, Venning M, Gokal R. Does CAPD guarantee adequate dialysis delivery and nutrition? Nephrol Dial Transplant. 1994;9:1721–1723.
- Rocco MV, Yan G, Gassman J, . Hemodialysis Study Group. Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. Am J Kidney Dis. 2002;39:146–153.
- Homma S, Hirose N, Ishida H, Ishii T, Araki G. Carotid plaque and intima-media thickness assessed by B-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke. 2001;32:830–835.
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28:53–61.