Abstract
Background: To investigate the renal microvascular injury in chronic aristolochic acid nephropathy (AAN) and the protective effects of Cozaar. Methods: Male Sprague-Dawley rats were randomized into three groups. Rats in the model group received Caulis Aristolochiae manshuriensis (CAM) decoction by gavage (10 mL/kg/day); those in the Cozaar group were gavaged with CAM and Cozaar (33.3 mg/kg/day); and those in the control group only received an equal daily volume of saline solution by gavage. Kidney tissues were observed under a light and electron microscope. CD34, caspase-3, and bone morphogenetic protein-7 (BMP-7) were determined by immunohistochemistry, and expressions of angiopoietin (Ang) 1 and 2, Tie-2, BMP-7, and vascular endothelial growth factor (VEGF) mRNA were monitored via real-time polymerase chain reaction (PCR). Results: (1) The kidney tissue injury in the chronic AAN model group was apparent, compared to the normal structure in the normal control group, and the Cozaar group showed relieved injury. (2) The expression of caspase-3 in the model group was elevated, while expressions of BMP-7 and CD34 were decreased (p < 0.05). Cozaar lessened caspase-3 expression (p < 0.05) and promoted BMP-7 and CD34 expressions (p < 0.05). (3) Real-time PCR demonstrated a downregulation of Ang-1, Tie-2, BMP-7, and VEGF mRNA (p < 0.05) and an upregulation of Ang-2 mRNA (p < 0.01) in the renocortex, while Cozaar upregulated the expression of Ang-1, Tie-2, BMP-7, and VEGF mRNA (p < 0.05). Conclusion: Renal microvascular injury was observed in chronic AAN, which was hypothetically correlated with a lack in the expressions of Ang-1, BMP-7, Tie-2, and VEGF and an excess in caspase-3 and Ang-2. Cozaar can significantly ameliorate the renal microvascular injury and protect renal function.
INTRODUCTION
Aristolochic acid nephropathy (AAN) is a rapidly progressing tubulointerstitial nephropathy induced by the aristolochic acid content in medicinal herbs. Growing number of reports in China have proved the disease to be a new challenge for nephrologists. However, its pathologic mechanism remains unclear, and an effective clinical drug therapy remains to be discovered. Present studiesCitation1 have demonstrated an ischemic response of the renal microvasculature and a loss of peritubular capillaries in AAN, but the nature of the injury remains controversial. Recently, Cozaar, one of the angiotensin II receptor blockers (ARBs), is generally accepted to be a renal protector, which has proved its benefits by limiting urine protein, decreasing blood pressure, and delaying the progress of chronic renal failure, particularly in the treatment of hypertensive nephropathy and diabetic nephropathy. This study is aimed to discover the renoprotective effect of Cozaar on a rat model of chronic AAN with Aristolochiae manshuriensis and to uncover the mechanism of microvascular injury and Cozaar’s interfering effect on it, thus investigating the renoprotective mechanism of Cozaar.
MATERIALS AND METHODS
Manshuriensis: Batch number: HY2003022002, origin of the northeast.
Manshuriensis decoction: Prepared by the Chinese medicine preparation department of the hospital: Manshuriensis pieces 20 kg, rinsed with water to remove dust and debris, plus water by 8 times the equivalent amount of herbs, were brought to boiling twice, the first 2 h and the second 1.5 h. The two liquids by filtration were merged into another liquid. The concentration of manshuriensis was 4 g/mL in the water extract. It was then salt packed in 500 mL bottles, sealed, sterilized with a flow of steam at 100°C for 30 min, and preserved at 4°C. The concentration of aristolochic acid A in the decoction is 0.26 mg/mL.
Cozaar (Hangzhou MSD Pharmaceutical Co., Ltd, Hangzhou, PR China): Specification: A Cozaar solution (containing Cozaar 3.33 mg/mL, lot number: 07115) was prepared with saline solution in proportion.
Reagents Biochemical testing reagents
The reagents used were urinary N-Acetyl-beta-D-glucosaminidase (NAG) ELISA kit (Wuhan EIAab Science Co., Ltd, Wuhan, PR China, batch number: E0069r) and urinary β2-MG ELISA kit (Rapidbio (RB), West Hills, CA, USA, batch number: 05090701).
Immunohistochemistry reagents
CD34 immunohistochemistry kit (lot number: F2018) was purchased from Haibo Valley Biological Technology Co., Ltd, Shanghai, PR China. Immunohistochemistry reagents bone morphogenetic protein-7 (BMP-7) (lot number: B1004C) and caspase-3 (batch number: C5407A) were purchased from Shanghai Canton Real Biotechnology Company, Shanghai, PR China.
Real-time PCR detection kit
Trizol total RNA extraction kit, rat GAPDH, angiopoietin (Ang) 1 and 2, Tie-2, BMP-7, vascular endothelial growth factor (VEGF) primers, and probe were provided by Shinegene Molecular Biotechnology Co., Ltd, Shanghai, PR China; RNA and quantitative RT-PCR (polymerase chain reaction) kit (BK801) were purchased from TaKaRa Bio Inc., Otsu, Shiga, Japan.
Male Sprague-Dawley rats are randomized into three groups: within the period of 21 weeks, rats in the model group received A. manshuriensis decoction by gavage (10 mL/kg/day); those in the Cozaar group were gavaged with Caulis Aristolochiae manshuriensis (CAM) and Cozaar (33.3 mg/kg/day); and those in the control group only received an equal daily volume of saline solution by gavage. The rats in each group were weighed once every week and the doses of saline solution and Cozaar were adjusted accordingly.
Urine Analysis and Blood Samples
At the end of 21 weeks, a 24 h urine sample was collected and the urine protein was determined by the automatic biochemistry analyzer. NAG and β2-MG were assessed with an ELISA kit. The rats were anesthetized before the surgical dissection and blood sample from the aorta was taken to evaluate serum creatinine (Scr), blood urea nitrogen (BUN), RBC, and Hb.
Histological Analysis of Kidney Tissue Injury
Light microscope
The kidney tissues were fixed in 10% buffered formalin and dehydrated, embedded in paraffin wax, and sliced into 3 μm sections. Pathology observation was performed after hematoxylin–eosin staining.
Electron microscope
The fresh kidney tissues were fixed in pre-cooling 2.5% glutaraldehyde and post-fixed in 1% osmium tetroxide solution, dehydrated by serial ethanol, and sliced into ultrathin sections. Renal ultrastructure was observed under an electron microscope (Philips, Tecrai 10, Dutch) after uranyl acetate and lead citrate double staining.
Immunohistochemistry
Slides of 3 μm thick sections were dewaxed to water, and endogenous enzyme action was closed by methanol peroxide. Immunohistochemistry was performed using StreptAvidin-Biotin Complex method, stained with Diaminobenzidine. Phosphate buffered saline buffer was used instead of primary antibody as a negative control (brown-yellow as positive). We used MIQAS medical imaging analyzing system (six specimens in each group). Three positive fields were randomly selected under a 250× light microscope, and the results were analyzed using positive staining relative area method: the relative positive tubulointerstitial area (%) = positive tubulointerstitial area/total vision field area × 100%.
Real-Time Fluorescent Quantitation PCR
To detect the expressions of Ang-1, Ang-2, Tie-2, BMP-7, and VEGF mRNA in the renal cortex of each group, 50–100 mg renal cortex tissues were ground into powder using nitrogen and extracted with Trizol method of total RNA, and was then detected with a UV spectrophotometer (A260/A280 ratio, 1.8–2.0). Reverse transcription of 1000 ng RNA into cDNA was performed for 15 min at 37°C and for 5 s at 85°C. PCR amplification of prepared cDNA under 20 μL reaction system was done under the following conditions: (1) pre-degeneration: 95°C, 10 s; (2) PCR reaction: 95°C, 5 s; 60°C, 31 s, 40 cycles. Data were analyzed using equipment accompanying software (ABI PRISM 7300, SDS Software (Applied Biosystems, Foster City, CA, USA)). Rat GADPH was used as the internal reference. The relative RNA expression levels were calculated as relative mRNA expression = 2–ΔCt, where ΔCt = target gene Ct – GAPDH Ct. The primer sequences are shown in .
Table 1. Primer sequences of amplified genes.
Table 2. Differences of body weight (BW), 24 h urine protein, and urine NAG between different groups ().
Table 3. Differences of Scr, BUN, Hb, and RBC between different groups ().
Statistical Analysis
Data were processed by SPSS 12.0 software (SPSS Ltd., Chicago, IL, USA) (). Differences of means among different groups were analyzed by one-way analysis of variance. Least significant difference test was used for multiple comparisons if variances were homogeneous among different groups, whereas Games–Howell was employed if they were nonhomogeneous. Linear correlation analysis was applied to determine whether there is a relationship between two sets of variables. p < 0.05 was considered as statistically significant.
RESULTS
Body Weight Change and Biochemical Indicators of Urine and Blood
General states and body weight change
The rats in the model appeared in a less active, lethargic, and less appetite state, progressing with a dried coat color accompanied by piloerection, localized depilation, and diarrhea. As the experiment proceeded, there was a stable increase in the body weight of the rats in the control group, while a slow increase in the model group and Cozaar group. At the end of the experiment, there was a significant decrease in the body weight of the rats in the model group, as compared to the control group (p < 0.01), and no significant difference was found between the Cozaar group and the model group (p > 0.05).
Urine protein, NAG enzyme, and β2-MG
24 h urine protein. The rats in the model group showed a higher urine excretion of protein in 24 h than did those in the control group (p < 0.01), while the Cozaar group demonstrated a significant decrease in protein urine, which indicated a possible renoprotective role of Cozaar by reducing the protein excretion ().
24 h NAG enzyme. The rats in the model group showed a significant increase in 24 h NAG as compared to the model group (p < 0.01), while the Cozaar group demonstrated a reduction in the level of NAG (p < 0.01). This finding may indicate that the elevated level of NAG was attributed to the renal tubule injury induced by aristolochic acid, and that Cozaar showed a protective effect on renal tubule injury by lowering NAG excretion ().
Urine β 2-MG. The rats in the model group showed a higher urine excretion of β2-MG in 24 h than did those in the control group (p < 0.05), and no statistically significant difference was shown between the Cozaar group and the model group (p > 0.05) (see ).
Blood levels of Scr, BUN, Hb, and RBC
At the 21st week, there was a significant difference in the elevated levels of Scr and BUN compared to the control group (p < 0.01), and Cozaar lowered BUN level (p < 0.01) but revealed little effect on Scr level (p > 0.05). The Hb and RBC levels were reduced in the model group (p < 0.01), and no significant difference was found between the Cozaar group and the model group (p > 0.05) (see ).
Histological Analysis of the Kidney Tissue and Immunohistochemistry
Histological analysis of the kidney tissue
Light microscopy. Twenty-one weeks after administration of Cozaar, the structure of the renal tubule and the interstitium was found normal in the control group. In the model group, degenerated, necrotic, and sloughed tubule epithelial cells, which were also seen in the Cozaar group, an exposed basement membrane, and some of the tubule structures were atrophied and lost, with moderate fibrosis of the interstitium. The Cozaar group demonstrated a less severe interstitial fibrosis ( and ).
Figure 1. Hematoxylin–eosin staining in rat renal tissue samples at week 21 (×400). (A) There was no significant histological abnormality in the control group. (B) Degenerated, necrotic, and sloughed tubule epithelial cells and an exposed basement membrane were seen, and some of the tubule structures were atrophied and lost. (C) In Cozaar group, only local mild tubular epithelial lesions were observed.
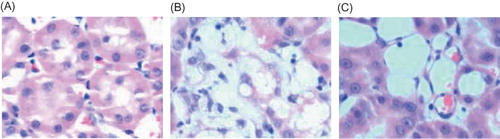
Figure 2. Masion staining in rat renal tissue samples at week 21 (×400). (A) There was no fibrosis of the interstitium in the control group. (B) A moderate fibrosis of the interstitium was seen in the model group. (C) A less severe interstitial fibrosis was observed in Cozaar group.
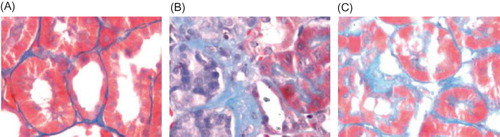
Figure 3. Electron microscopy of glomerulus at week 21 (×4200). (A) The ultrastructure of intact glomerulus was seen in the control group. (B) An ischemic shrinkage of the glomerular basement membrane was found in the model group. (C) A less severe ischemic shrinkage of the glomerular basement membrane was observed in Cozaar group.
Electron microscopy. Twenty-one weeks after administration of Cozaar, we found an intact glomerulus with normal structures of the tubule epithelial cells, the basement membrane, and the interstitium in the control group. The model group displayed an ischemic shrinkage of the glomerular basement membrane, thickening and collapse of the tubule basement membrane, and a significant amount of fasciculation of collagen fibers at the affected interstitium. Cozaar treatment showed less severe ischemic shrinkage of the glomerular basement and less fasciculations of collagen fibers at the affected interstitium ().
Immunohistochemistry assay of the kidney tissue
In the control group, BMP-7 was largely expressed in the renal tubular epithelial cells and renal interstitium, especially in the medulla area, while it was decreased in the model group (p < 0.01). After Cozaar treatment, BMP-7 expression in the renal tissue was significantly increased as compared with the model group (p < 0.05) (, ).
In the control group, caspase-3 was less expressed in the renal tubular interstitium, while it was increased in the model group, especially in the injured renal tubules (p < 0.05). After Cozaar treatment, caspase-3 expression in the renal tissue was significantly decreased as compared with the model group (p < 0.05) (, ).
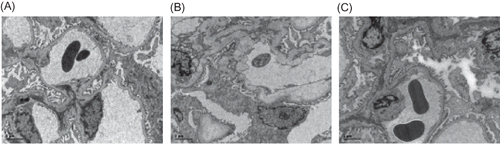
Figure 4. Electron microscopy of rat renal tubule at week 21 (×11,500). (A) The normal tubule epithelial cells and basement membrane were seen in the control group. (B) Thickening and collapse of the tubule basement membrane were found in the model group. (C) A mild thickening and collapse of the tubule basement membrane was observed in Cozaar group.
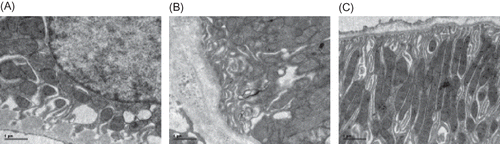
Figure 5. Electron microscopy of rat renal interstitium at week 21 (×11,500). (A) The normal ultrastructure of renal interstitium was seen in the control group. (B) A significant amount of fasciculation of collagen fibers at the affected interstitium was found in the model group. (C) Less fasciculations of collagen fibers at the affected interstitium were observed in Cozaar group.
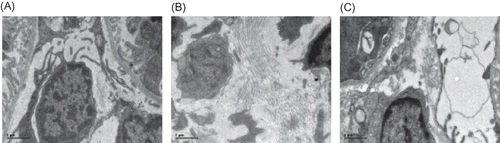
Figure 6. BMP-7 expression in rat renal tissue at week 21 (×250). (A) BMP-7 was largely expressed in the renal tubular epithelial cells and renal interstitium, especially in the medulla area in the control group. (B) A decreased BMP-7 expression was found in the renal tubular epithelial cells and renal interstitium. (C) BMP-7 expression in the renal tissue was significantly increased in Cozaar group.
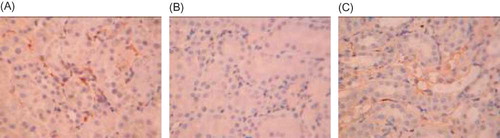
Table 4. Differences of relative positive area of BMP-7, caspase-3, and CD34 expressions in rat renal tissue between different groups ().
In the control group, CD34 was largely expressed in the renal tubular interstitium, while it was decreased in the model group (p < 0.01). After Cozaar treatment, CD34 expression in the renal tubular interstitium was significantly increased as compared with the model group (p < 0.05), suggesting the renal interstitium microvessels were significantly decreased in the model group, and the microvascular injury was decreased by Cozaar treatment (, ).
Results of Real-Time PCR
The mRNA expressions of Ang-1, Tie-2, BMP-7, and VEGF of renal cortex in the model group were significantly reduced as compared with the normal control group (p < 0.01). After Cozaar intervention, the mRNA expressions of these cytokines were significantly upregulated (p < 0.01). Conversely, the mRNA expression of Ang-2 of renal cortex in the model group was significantly increased as compared with the normal control group (p < 0.01), but no significant difference was found between the Cozaar group and the model group (p > 0.05) ().
Figure 7. Caspase-3 expression in rat renal tissue (×250). (A) Caspase-3 was less expressed in the renal tubular interstitium in the control group. (B) An increased caspase-3 expression was found in the renal interstitium in the model group. (C) Caspase-3 expression in the renal tissue was significantly decreased in Cozaar group.
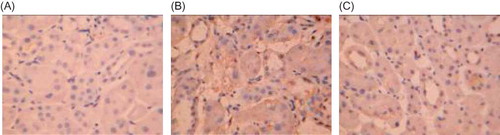
Figure 8. CD34 expression in rat renal tissue (×250). (A) CD34 was largely expressed in the renal tubular interstitium in the control group. (B) A decreased CD34 expression was found in the renal interstitium in the model group. (C) CD34 expression in the renal tissue was significantly increased in Cozaar group as compared with the model group.
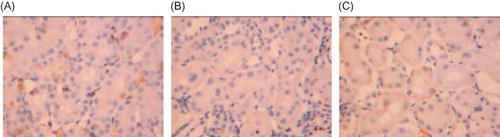
Table 5. Differences of Ang-1, Ang-2, Tie-2, and VEGF mRNA expressions between different groups ().
Correlation among the Expressions of CD34, Ang-1, Ang-2, Tie-2, and VEGF
The correlation analyses showed that the CD34 expression in the renal tissue of chronic AAN model was positively correlated with the mRNA expressions of Ang-1, Tie-2, and VEGF mRNA (r = 0.796, r = 0.8, r = 0.817, respectively, p < 0.01) and negatively correlated with the mRNA expression of Ang-2 (r = –0.653, p < 0.01). These results suggested that the injury or loss of microvessels in renal interstitium was closely correlated with expressions of cytokines such as Ang-1, Ang-2, Tie-2, and VEGF.
DISCUSSION
The pathogenesis of AAN is unclear. Recent studiesCitation1–3 have shown that aristolochic acid can cause renal interstitial capillary changes, which mainly appear as thickening of wall and lumen stenosis. Some micro-arteries are occluded and present kidney ischemic changes. Density of peritubular capillaries was significantly decreased, and peritubular capillary lesions were focally distributed. The lumen of peritubular capillaries in the severely damaged, inflammatory infiltrated, and fibrosis area was evidently narrowed or deformed, suggesting the pathogenesis of AAN may have relevance to the microvascular damage and loss.
Loss and damage to the kidney microvasculature are related to the imbalance between the focal angiogenic and anti-angiogenic factors.Citation4 Ang is a newly discovered protein family which may collaborate with VEGF in angiogenesis. The family contains Ang-1, Ang-2, Ang-3, and Ang-4, the specific receptors of which are Tie-1 and Tie-2. Currently, Ang-1, Ang-2, and Tie-2 are relatively better understood.Citation5–7 Ang-1 is mainly secreted by smooth muscle cells, which act through Tie-2 to regulate the angiogenesis and endothelial progenitor cells stabilization. Overexpression of Ang-1 can antagonize the vessel leakageCitation8 caused by inflammatory factors (platelet-activating factors, etc.) and mustard oil. Ang-2 functions primarily through competitive inhibition of Ang-1 so that the unstable and leakage vessels may form. When VEGF is lacking in the focal area, Ang-2 can induce phosphorylation and degeneration of Tie-2, resulting in breakage and decrease in amounts of the vessels. However, in the presence of VEGF, Ang-2 can promote angiogenesis.Citation9,10 VEGF is an angiogenesis regulator, which is related to the promotion of vascular endothelial cell proliferation, an increase in microvascular permeability, the induction of angiogenesis, and other functions.Citation11
CD34 is a single-chain transmembrane glycoprotein about 110 kD, which is mainly expressed in vascular endothelial cells.Citation12 Among the markers of vascular endothelial cells, CD34 is more sensitive, specific, and also easy to be observed.Citation13 Since CD34 is the marker of vascular endothelial cells, where positive staining can reflect the distribution of capillaries,Citation1 we use the results of CD34 immunohistochemical staining to indirectly reflect microvascular damage of kidney tissue.
We found a significant reduction in the renal microvessel between the model group and the normal group at 21 weeks. This suggests the existence of microvessel damage in chronic AAN, which is also consistent with the literature in recent years.Citation1,14 In this process, we found significantly reduced levels of Ang-1, Tie-2, and VEGF mRNA expression and increased Ang-2 expression in the kidneys. Correlation analysis also shows the indexes mentioned above are closely related to microvascular damage. Hence, we infer that the downregulation of Ang-1, Tie-2, and VEGF mRNA may result in impaired renal microvascular regeneration. Meanwhile, increased expression of Ang-2 mRNA antagonized the Ang-1-induced angiogenesis. Also, the lack of renal VEGF led to a major physiological role of Ang-2 to promote the atrophy of blood vessels. These factors ultimately lead to the renal microvascular loss and damage.
Renal microvascular loss and damage will result in renal tubular interstitial ischemia, hypoxia, and nutritional disorders, which may cause necrosis or apoptosis to tubular epithelial cells, and decreased regeneration and repair capacity, further leading to renal interstitial fibrosis. Current studies reportCitation15 that caspase-3 and BMP-7 may play an important role in the apoptosis of renal tubular cells. A variety of factors are involved in apoptosis signaling pathways, and caspases in apoptosis protease cascade stand in a central place: caspase-3 is one of the core proteins, with its key role in the implementation of apoptosis.Citation16,17 BMP-7 is a subgroup in the transforming growth factor β (TGF-β) superfamily, maintaining the structure and function of the kidneys.Citation18 BMP-7, as a negative regulator of fibrosis, has a unique restoration and maintenance function of epithelial cells, inhibits renal epithelial apoptosis, reduces the expression of many proinflammatory factors, activates extracellular matrix degradation, and affects TGF-β/Smads pathway and the reciprocal inhibition with TGF-β.Citation19,20 In recent years, animal studiesCitation21,22 found that certain kidney diseases associate with reduced BMP-7, and a certain dose of BMP-7 can reduce kidney damage.
Using immunohistochemistry to investigate the expression of caspase-3 and BMP-7 in the renal tissue with AAN demonstrated significant elevated caspase-3 expression (p < 0.05) and lowered BMP-7 expression (p < 0.01), indicating an overexpression of caspase-3, while the expression of BMP-7, a factor that inhibits apoptosis and fibrosis, was downregulated. As a consequence, renal tubular injury mainly caused by the apoptosis of epithelial cells and impaired auto-repair capability eventually participates in the development of fibrosis.
Recently, Cozaar, as one of the ARBs, is generally accepted to be a renal protector, which has shown its benefits by limiting urine protein, decreasing blood pressure, and delaying the progress of chronic renal failure, particularly in the treatment of hypertensive nephropathy and diabetic nephropathy. So here we attempted to investigate whether a suspected renoprotective effect exists in chronic AAN.
With A. manshuriensis, our study copied the rat model of chronic AAN. We found a statistically significant increase in urine protein, NAG, and β2-MG in the model group during the experiment, while after the administration of Cozaar, the levels of urine protein and NAG were decreased, which may indicate that Cozaar improved the tubular injury in chronic AAN. Twenty-one weeks after administration of Cozaar, histological analysis of the kidney tissue injury under light and electron microscopes demonstrated a normal structure of the renal tubule and the interstitium. In the model group, degenerated, necrotic, and sloughed tubule epithelial cells, an exposed basement membrane, and some of the tubule structures were atrophied and lost, with large amounts of fasciculations of collagen fibers in the interstitium. Cozaar administration showed improved glomerular basement ischemic shrinkage, tubule epithelial cell degeneration and necrosis, and interstitial fibrosis. These results indicate that Cozaar may, to some extent, ease the renal tissue morphological change in chronic AAN. Other results proved Cozaar to alleviate the microvascular injury in the renal tissue, hypothetically by upregulating the expression of Ang-1, Tie-2, and VEGF mRNA.
As a summary, the microvascular injury, tubular necrosis, and apoptosis in chronic AAN may have a correlation with inadequate expression of Ang-1, BMP-7, Tie-2, and VEGF and overexpression of caspase-3 and Ang-2. Consequently, the multifaceted factors co-initiated and promoted the development of fibrosis. On the other hand, Cozaar alleviated the microvascular injury by means of upregulating Ang-1, Tie-2, and VEGF mRNA expression and may have proved its renoprotective role in AAN.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Sun D, Feng JM, Dai C, . Influence of hypoxia caused by impairment of peritubular capillary on the progression of chronic aristolochic acid nephropathy. Zhonghua Yi Xue Za Zhi. 2006;86(21):1464–1469.
- Sun D, Liu CX, Ma YY, Zhang L. Protective effect of prostaglandin e1 on renal microvascular injury in rats of acute aristolochic acid nephropathy. Ren Fail. 2011;33(2):225–232.
- Wen YJ, Qu L, Li XM. Ischemic injury underlies the pathogenesis of aristolochic acid-induced acute kidney injury. Transl Res. 2008;152(1):38–46.
- Flyvbjerg A. Putative pathophysiological role of growth factors and cytokines in experimental diabetic kidney disease. Diabetologia. 2000;43:1205–1223.
- Wu X, Liu N. The role of Ang/Tie signaling in lymphangiogenesis. Lymphology. 2010;43(2):59–72.
- Thomas M, Augustin HG. The role of the angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12(2):125–137.
- Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312(5):630–641.
- Fukuhara S, Sako K, Noda K, Zhang J, Minami M, Mochizuki N. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol Histopathol. 2010;25(3):387–396.
- Yancopoulos GD, Davis S, GaleN W, . Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248.
- Holash J, Maisonpierre PC, Compton D, . Vessel cooption, regression,, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998.
- Kang DH, Anderson S, Kim YG, . Impaired angiogenesis in the aging kidney: Vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis. 2001;37(3):601–611.
- Civin CI, Strauss LC, Brovall C, . Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1acells. J Immunol. 1984;133:157–165.
- Schoell WM, Pieber D, Reich O, . Tumor angiogenesis as a prognostic factor in ovarian carcinoma: Quantification of endothelial immunoreactivity by image analysis. Cancer. 1997;80:2257–2262.
- Sun D, Sun L, Wang W, . Effects of ginkgo biloba extract on peritubular capillaries in rats of aristolochic acid nephropathy. Zhong Guo Xian Dai Yi Xue Za Zhi. 2006;16(3):346–350, 353.
- Zeisberg M, Shah AA, Kalluri R. Bonemorphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. Biol Chem. 2005;280(9):8094–8100.
- Tumane RG, Pingle SK, Jawade AA, Nath NN. An overview of caspase: Apoptotic protein for silicosis. Indian J Occup Environ Med. 2010;14(2):31–38.
- Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351(1–2):41–58.
- Kazama I, Mahoney Z, Miner JH, Graf D, Economides AN, Kreidberg JA. Podocyte-derived BMP7 is critical for nephron development. J Am Soc Nephrol. 2008;19(11):2181–2191.
- Xu YF, Wan JX, Jiang DW. Effects of bone morphogenic protein-7 on transdifferentiation and the expression of connective tissue growth factor of human renal tubular epithelial cells induced by transforming growth factor-beta1. Zhonghua Yi Xue Za Zhi. 2009;89(23):1639–1644.
- Dudas PL, Argentieri RL, Farrell FX. BMP-7 fails to attenuate TGF-beta1-induced epithelial-to-mesenchymal transition in human proximal tubule epithelial cells. Nephrol Dial Transplant. 2009;24(5):1406–1416.
- Negri AL. Prevention of progressive fibrosis in chronic renal diseases: Antifibroticagents. J Nephrol. 2004;17(4):496–503.
- Dube PH, Almanzar MM, Frazier KS. Osteogenic protein-1: Gene expression and treatment in rat remnant kidney model. Toxicol Pathol. 2004;32(4):384–392.
