Abstract
Introduction: Coronary heart disease (CHD) is the leading cause of death in hemodialysis (HD) patients. Inflammation contributes to the pathogenesis of atherosclerosis in this population. Indoleamine 2,3-dioxygenase (IDO), an enzyme with immunomodulatory properties, was evaluated in HD patients with or without CHD. Methods: Of the total of 66 HD patients, 22 of them with CHD were confirmed by coronary angiography and 24 healthy volunteers were enrolled in the study. Plasma IDO was assessed by means of enzyme-linked immunosorbent assay. Serum interleukin-6 (IL-6) and C-reactive protein (CRP) were also measured. Results: Compared with healthy volunteers, plasma IDO concentration was markedly increased in HD patients (median 8.04 ng/mL vs. 48.9 ng/mL). Serum IL-6 and CRP were also significantly increased in HD patients. Compared with HD patients without CHD, plasma IDO concentration was significantly increased in HD patients with CHD (median 38.6 ng/mL vs. 74.5 ng/mL). Neither IL-6 nor CRP differed between the last two groups. IDO was negatively correlated with IL-6 and CRP. Conclusion: IDO concentration is increased in HD patients and is increased further in HD patients with CHD. It remains to be elucidated if increased IDO plays a direct role in the pathogenesis of atherosclerosis or if it affects atherosclerosis indirectly by curtailing chronic inflammation or both.
INTRODUCTION
Cardiovascular disease, and mainly coronary heart disease (CHD), is the leading cause of death in hemodialysis (HD) patients.Citation1 However there is an apparent different relationship, called “reverse epidemiology,” between numerous known risk factors established in general population and outcomes in HD patients.Citation2 Thus, the research for pathogenesis and markers of CHD in HD patients continues.
Recently, some studies evaluated the role of indoleamine 2,3-dioxygenase (IDO) in the pathogenesis of atherosclerosis in HD patients.Citation3–6 IDO is a 45 kDa enzyme that catalyzes the initial rate-limiting step of tryptophan degradation along the kynurenine pathway. IDO, which is inducible by various inflammatory stimuli, is widely distributed in various immune and nonimmune cell types, and downregulates adaptive immune response and inflammation as well.Citation7–10 Recently, we have shown that IDO plays a significant role in the impaired adaptive immunity that characterizes HD patients.Citation11
Considering that inflammation contributes to atherosclerosis progression in HD patients,Citation12 evaluation of a possible role of this immunomodulatory enzyme is interesting. Currently, it is known that IDO is expressed in the atherosclerotic plaques in humansCitation13 and some studies suggest that IDO affects directly the development of atherosclerosis.Citation14–17 The available studies in HD patients showed increased IDO activity, which was increased further in HD patients with atherosclerotic lesions.Citation3–6 However, these studies evaluated IDO activity very indirectly by estimating the serum kynurenine to tryptophan ratio. Such an approach is rather inaccurate because it does not consider the low serum tryptophan levels in case of malnutrition, the decreased renal excretion of kynurenine pathway products, and the activity of the liver tryptophan 2,3-dioxygenase, which is upregulated in experimental models of renal failure.Citation18,19
In this study the role of IDO in the pathogenesis of CHD in HD patients was evaluated by assessing for first time plasma IDO at the protein level. Serum interleukin-6 (IL-6) and C-reactive protein (CRP) were also measured because inflammation usually accompanies HD,Citation20–22 has been incriminated for atherosclerosis progression in this population,Citation12 and upregulates IDO.Citation7,8
PATIENTS AND METHODS
Patients
Sixty-six stable HD patients (61.06 ± 12.66 years) and twenty-four healthy volunteers derived from two HD units personnel (mean age 57.0 ± 8.61 years) participated in the study. The two groups did not differ significantly regarding age (p = 0.09, unpaired t-test). Of the total of 66 patients, 22 of them who suffered from CHD were confirmed by coronary angiography, while the other 44 were asymptomatic. The two groups of patients did not differ significantly regarding age (59.79 ± 11.56 years vs. 60.27 ± 11.07 years, p = 0.873, unpaired t-test).
Because asymptomatic CHD is usual in HD patients,Citation23 we left 2.5 years to pass between the blood collection and the preparation of this article. If during this period an asymptomatic subject developed symptoms of CHD, he/she was included in the relative group. This excludes to a great extent the existence of clinically significant coronary artery stenosis at the time of blood collection, that is, in a patient or healthy volunteer who remained asymptomatic during this 2.5-year period.
All patients were anuric and the cause of end-stage renal disease was diabetes mellitus in 22 patients, primary glomerulonephritis in 19 patients, obstructive nephropathy in 4 patients, hypertension in 4 patients, interstitial nephritis in 4 patients, autosomal dominant polycystic kidney disease in 3 patients, Alport’s syndrome in 1 patient, and unknown in 9 patients. Patients underwent regular HD with low-flux poly-sulfone dialyzers (Flow-flux series; Fresenius Medical Care, Bad Homburg, Germany) for 4-h sessions, three times a week and for at least 1 year prior to the study. None of the patients or healthy volunteers suffered from any infection, malignancy, or autoimmune disease. Finally, none of the patients was receiving cytotoxic drugs, corticosteroids, or nonsteroidal anti-inflammatory drugs. An informed consent was obtained from each individual enrolled in the study and the Hospital Ethics Committee gave its approval to the study protocol.
Methods
Blood samples were drawn just before the start of the second HD session of the week. The samples were centrifuged immediately and the harvested plasma and serum were stored at −20°C.
Plasma IDO levels were assessed by means of enzyme-linked immunosorbent assay (ELISA) (CUSABIO Biotech Co., Wuhan, China). The analytical limit of detection of the above ELISA kit is 0.195 ng/mL. Serum IL-6 was assessed by means of a commercially available ELISA kit with sensitivity less than 2 pg/mL (BioSource Europe S.A., Nivelles, Belgium). Serum CRP was measured using the COBAS INTEGRA 400 automatic analyzer (Roche Diagnostics GmbH, Mannheim, Germany).
Statistical Analysis
For comparisons between HD patients and healthy volunteers or between HD patients with or without CHD Mann–Whitney U-test was used. The results were expressed as median and range and a p < 0.05 was considered statistically significant. Spearman’s correlation test was used for evaluating correlations.
RESULTS
IDO, IL-6, and CRP in HD Patients and Healthy Volunteers
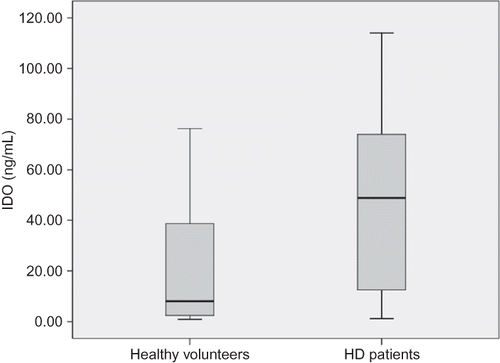
Compared with healthy volunteers, plasma IDO concentration was markedly increased in HD patients (median 48.9 ng/mL; range 112.82 ng/mL vs. median 8.04 ng/mL; range 75.46 ng/mL, p = 0.002) (). Serum IL-6 was significantly higher in HD patients as well (median 10 pg/mL; range 53.3 pg/mL vs. median 2.2 pg/mL; range 6.7 pg/mL, p < 0.001). Serum CRP was also increased in HD patients (median 4.68; range 72.1 mg/L vs. median 3.0 mg/L; range 2.94 mg/L, p = 0.001).
Figure 1. Plasma indoleamine 2,3-dioxygenase (IDO) concentrations in hemodialysis (HD) patients and healthy volunteers.
Note: Compared with healthy volunteers, plasma IDO concentration was markedly increased in HD patients (median 48.9 ng/mL; range 112.82 ng/mL vs. median 8.04 ng/mL; range 75.46 ng/mL).
IDO, IL-6, and CRP in HD Patients with or without CHD
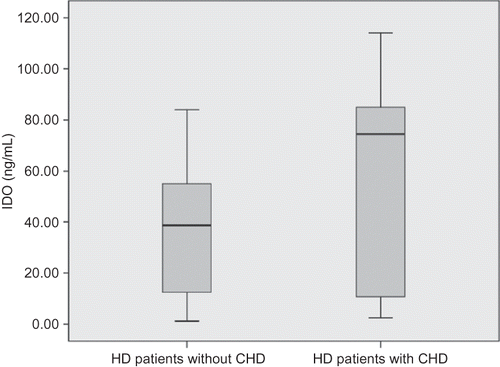
Compared with HD patients without CHD, plasma IDO concentration was significantly increased in HD patients with CHD (median 74.5 ng/mL; range 111.5 ng/mL vs. median 38.6 ng/mL; range 82.82 ng/mL, p = 0.006) (). Serum IL-6 levels did not differ between HD patients without or with CHD (median 10 pg/mL; range 52.2 pg/mL vs. median 10 pg/mL; range 33.6 pg/mL, p = 0.662). CRP did not differ between HD patients without or with CHD as well (median 5.24 mg/L; range 42.8 mg/L vs. median 3.0 mg/L; range 72.1 mg/L, p = 0.178).
Figure 2. Plasma indoleamine 2,3-dioxygenase (IDO) concentration in hemodialysis (HD) patients with or without coronary heart disease (CHD).
Note: Compared with HD patients without CHD, plasma IDO concentration was significantly increased in HD patients with CHD (median 74.5 ng/mL; range 111.5 ng/mL vs. median 38.6 ng/mL; range 82.82 ng/mL).
Correlations among IDO, IL-6, and CRP in HD Patients
In HD patients IDO was negatively correlated with IL-6 (ρ = −0.253, p = 0.04) and CRP (ρ = −0.348, p = 0.004). Interestingly, IL-6 was positively correlated with CRP (ρ = 0.607, p < 0.001), which was expected since IL-6 is the major inducer of CRP production in the liver.Citation24 IDO, IL-6, and CRP levels did not differ between diabetics and nondiabetic HD patients (data not shown).
DISCUSSION
Compared with healthy volunteers, plasma IDO concentration was revealed to be markedly increased in HD patients. Considering that IDO is inducible by various inflammatory stimuli,Citation7–10 the above finding could be the result of the chronic inflammation that characterizes this population.Citation20–22 The increased serum IL-6 and CRP levels in HD patients detected in this study reconfirm that HD should be considered as an inflammatory condition.
Compared with HD patients without CHD, plasma IDO concentration was significantly increased in HD patients with CHD. Our study confirms for the first time that circulating IDO at the protein level is increased in HD patients and is increased further in HD patients with CHD. This is in accordance with the results of previous studies that showed increased IDO activity in HD patients, which was increased further in HD patients with atherosclerotic lesions.Citation3–6 Currently, it is known that IDO is expressed in atherosclerotic plaques in humansCitation13 and some studies suggest that IDO affects directly the development of atherosclerosis. IDO activity is increased in patients with CHD and normal renal functionCitation14 and is correlated with carotid intima–media thickness in young females.Citation15 Additionally, increased kynurenine to tryptophan ratio is associated with an increased risk for a major coronary event or death in patients with stable CHD.Citation16 However, IDO activity has been found to be lower in smokers, that is, in a situation that promotes atherosclerosis.Citation25 Experimentally kynurenine increases adhesion of leukocytes to vascular endothelium under flow conditions,Citation17 but it protects endothelium in hyperhomocysteinemia,Citation26 decreases blood pressure, and relaxes coronary arteries.Citation27
On the other hand, increased IDO expression in HD patients with CHD could simply be the result of the chronic inflammation that characterizes this population.Citation20–22 Chronic inflammation is also known to contribute to the pathogenesis of atherosclerosis in HD patients.Citation12 The lack of difference in the inflammatory markers IL-6 and CRP between HD patients with or without CHD detected in this study could reflect the effect of IDO. It is known that after its expression due to inflammatory stimuli, IDO can accelerate its own production forming positive feedback loopsCitation28,29 that could counteract inflammation, for example, through amelioration of oxidative stress by certain kynurenine pathway productsCitation9 or by inhibiting regulatory T-cell conversion to proinflammatory helper T-cell type 17.Citation10 The negative correlation between IDO and IL-6, as well as between IDO and CRP detected in this study, is in accordance with the above scenario. Thus IDO could indirectly protect against atherosclerosis by curtailing inflammation. The increased IDO levels in HD patients with CHD detected in this study could represent a greater activation of this anti-inflammatory compensation mechanism.
In conclusion, IDO concentration is increased in HD patients and is increased further in HD patients with CHD. It remains to be elucidated if increased IDO plays a direct role in the pathogenesis of atherosclerosis or if it affects atherosclerosis indirectly by curtailing chronic inflammation or both.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10:1606–1615.
- Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808.
- Koenig P, Nagl C, Neurauter G, Schennach H, Brandacher G, Fuchs D. Enhanced degradation of tryptophan in patients on hemodialysis. Clin Nephrol. 2010;74:465–470.
- Schefold JC, Zeden JP, Fotopoulou C, . Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: A possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant. 2009;24:1901–1908.
- Pawlak K, Brzosko S, Mysliwiec M, Pawlak D. Kynurenine quinolinic acid—the new factors linked to carotid atherosclerosis in patients with end-stage renal disease. Atherosclerosis. 2009;204:561–566.
- Kato A, Suzuki Y, Suda T, . Relationship between an increased serum kynurenine/tryptophan ratio and atherosclerotic parameters in hemodialysis patients. Hemodial Int. 2010;14:418–424.
- King NJ, Thomas SR. Molecules in focus: Indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol. 2007;39:2167–2172.
- Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: Focus on hematology. Blood. 2009;113:2394–2401.
- Thomas SR, Stocker R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999;4:199–220.
- Sharma MD, Hou DY, Liu Y, . Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111.
- Eleftheriadis T, Liakopoulos V, Antoniadi G, Stefanidis I, Galaktidou G. Indolemanine 2,3-dioxygenase is increased in hemodialysis patients and affects immune response to hepatitis B vaccination. Vaccine. 2011;29:2242–2247.
- Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658.
- Niinisalo P, Oksala N, Levula M, . Activation of indoleamine 2,3-dioxygenase-induced tryptophan degradation in advanced atherosclerotic plaques: Tampere vascular study. Ann Med. 2010;42:55–63.
- Wirleitner B, Rudzite V, Neurauter G, . Immune activation and degradation of tryptophan in coronary heart disease. Eur J Clin Invest. 2003;33:550–554.
- Pertovaara M, Raitala A, Juonala M, . Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: The Cardiovascular Risk in Young Finns Study. Clin Exp Immunol. 2007;148:106–111.
- Pedersen ER, Midttun O, Ueland PM, . Systemic markers of interferon γ mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:698–704.
- Barth MC, Ahluwalia N, Anderson TJ, . Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J Biol Chem. 2009;284:19189–19195.
- Saito K, Fujigaki S, Heyes MP, . Mechanism of increases in L-kynurenine and quinolinic acid in renal insufficiency. Am J Physiol Renal Physiol. 2000;279:F565–F572.
- Pawlak D, Tankiewicz A, Matys T, Buczko W. Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J Physiol Pharmacol. 2003;54:175–189.
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007;20:440–451.
- Eleftheriadis T, Kartsios C, Yiannaki E, . Chronic inflammation and T cell zeta-chain downregulation in hemodialysis patients. Am J Nephrol. 2008;28:152–157.
- Eleftheriadis T, Kartsios C, Yiannaki E, . Chronic inflammation and CD16+ natural killer cell zeta-chain downregulation in hemodialysis patients. Blood Purif. 2008;26:317–321.
- Patsalas S, Eleftheriadis T, Spaia S, . The value of computed tomography-derived coronary artery calcification score in coronary artery disease detection in asymptomatic hemodialysis patients. Ren Fail. 2005;27:683–688.
- Kushner I. Regulation of the acute phase response by cytokines. Perspect Biol Med. 1993;36:611–622.
- Pertovaara M, Heliovaara M, Raitala A, Oja SS, Knekt P, Hurme M. The activity of the immunoregulatory enzyme indoleamine 2,3-dioxygenase is decreased in smokers. Clin Exp Immunol. 2006;145:469–473.
- Wejksza K, Rzeski W, Turski WA. Kynurenic acid protects against the homocysteine-induced impairment of endothelial cells. Pharmacol Rep. 2009;61:751–756.
- Wang Y, Liu H, McKenzie G, . Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med. 2010;16:279–285.
- Fallarino F, Grohmann U, You S, . The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761.
- Muller AJ, Sharma MD, Chandler PR, . Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3‐dioxygenase. Proc Natl Acad Sci USA. 2008;105:17073–17078.
