Abstract
Diabetic nephropathy (DN) is one of the main causes of end-stage renal disease. Many studies have indicated that transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF) were involved in the pathophysiological mechanisms of DN. In addition, quercetin has been suggested to attenuate DN. In this study, we aim to examine whether quercetin ameliorates renal function through an effect on the expressions of TGF-β1 and CTGF in streptozotocin (STZ)-induced diabetic rats. Diabetes was induced in Sprague–Dawley rats with a single intraperitoneal injection of STZ. The diabetic rats were then randomized to diabetic group and quercetin therapy group. At the end of the 12th week, blood glucose, body weight, kidney weight/body weight, urine albumin excretion (UAE), serum creatinine (sCr), blood urea nitrogen (BUN), and creatinine clearance (Ccr) were measured. The expressions of TGF-β1 and CTGF in the kidneys were determined using real-time PCR and Western blot method. Diabetic rats showed significant increases in blood glucose, kidney weight/body weight, UAE, sCr, BUN, and Ccr than control group. Treatment with quercetin improved these parameters except blood glucose. Compared with the control group, the expressions of TGF-β1 and CTGF were elevated in the diabetic group. The overexpressions of TGF-β1 and CTGF in the renal tissues of diabetic rats were attenuated by administration of quercetin. Our results suggest that quercetin improved renal function in rats with DN by inhibiting the overexpressions of TGF-β1 and CTGF in the kidney.
INTRODUCTION
Diabetic nephropathy (DN), one of the microvascular complications of diabetes, is the primary cause of chronic kidney disease and end-stage kidney disease. Both hemodynamic and metabolic factors have been implicated in the pathogenesis of DN. The mainstay for risk reduction in DN is intensive glycemic control and blood pressure control.Citation1,2 However, these therapeutic approaches are insufficient to control the progression of DN.Citation3 Therefore, there is a vital need to identify novel pathophysiologic pathways and explore novel therapeutic agents to ameliorate the induction and progression of DN.
The progression of DN is related to many factors including overproduction of transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF). TGF-β1 expression was found to be elevated in the glomeruli of streptozotocin (STZ)-induced diabetic rats.Citation4 TGF-β1 has been shown to play a key role in the development of hypertrophy and the accumulation of extracellular matrix (ECM) in diabetes.Citation5 Glomerular CTGF mRNA levels were demonstrated to be significantly increased in DN patients compared with normal controls.Citation6 CTGF could promote the deposition of ECM components like collagen I, collagen IV, and fibronectin and thus could enhance the disassembly and hypertrophy of mesangial cells.Citation7
Quercetin is the most common of the flavonoid aglycones found in the diet. Quercetin has a wide range of biological activity including antiviral, antimicrobial, anti-inflammatory, and anticancer effects.Citation8 Recent studies have shown that quercetin significantly inhibited the expression of TGF-β1 in bile duct ligation of rats and neointima in rat abdominal aorta.Citation9,10 In another study, the increased expression of CTGF mRNA in hepatic stellate cells was abrogated by quercetin.Citation11
Previous study has suggested that quercetin could attenuate DN through anti-oxidative mechanism in diabetic rats.Citation12 Quercetin is hypothesized to ameliorate renal function through other mechanisms. Therefore, the aim of this study is to determine whether quercetin ameliorates renal function through an effect on the expressions of TGF-β1 and CTGF in STZ-induced diabetic rats.
MATERIALS AND METHODS
Diabetic Rat Model
Male Sprague–Dawley rats (200–250 g) were purchased from National Rodent Laboratory Animal Resources (Shanghai Branch, Shanghai, China). Rats were maintained in an air-conditioned room (20 ± 1°C) with a normal night and day cycle. Rats were fed with semi-purified basal diet and demineralized drinking water ad libitum. The rats were allowed to acclimatize to the laboratory environment for a week before the start of the experiment. Diabetes was induced by a single intraperitoneal injection of STZ (Sigma, St. Louis, MO, USA) at 55 mg/kg body weight in 0.01 M citrate buffer (Sangon Biotech, Shanghai, China; pH 4.5) after 12 h of fasting. Age-matched control rats received an equal volume of vehicle (0.01 M citrate buffer, pH 4.5). Three days after STZ injection, tail vein blood glucose concentration was measured to confirm the induction of diabetes. Rats with a blood glucose level over 300 mg/dL were considered as diabetes-induced rats.
Groupings and Treatment Protocol
The rats were then randomized into three groups: (1) control group (n = 12) with nondiabetic control rats, (2) diabetic group (DM, n = 12), and (3) quercetin-treated diabetic group (n = 12). From 3 days after the initial STZ injection, the diabetic rats in quercetin-treated group were given quercetin (Sangon Biotech; 50 mg/kg) by intragastric administration once a day for 12 weeks. The control and DM groups received vehicle alone.
Biochemical Measurements
After 12 weeks of treatment, rats were placed in individual metabolic cages to collect 24-h urine specimens. Urine samples were then analyzed for levels of urine albumin excretion (UAE). Blood was obtained from the tail vein. Serum creatinine (sCr) and blood urea nitrogen (BUN) levels were determined by standard methods using an autoanalyzer (Hitachi 7600, Tokyo, Japan). UAE was determined by immunoturbidimetry (Hitachi 7020, Japan). Creatinine clearance (Ccr) (in mL/min/kg body weight) was also calculated. All procedures were performed according to the guidelines for Animal Experiments at Fujian Medical University.
Real-Time PCR
Total RNA was isolated from whole kidney using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The first strand cDNA synthesis was carried out by using a reverse transcription system kit according to the instructions of the manufacturer (Takara, Otsu, Japan). To evaluate the relative levels of mRNA for TGF-β1 and CTGF, we performed real-time reverse transcription PCR using a SYBR Green kit (Takara) on the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA). The primers were as follows: forward for TGF-β1, 5′-GAGAGCCCTGGATACCAACTACTG-3′; reverse for TGF-β1, 5′-GTGTGTCCAGGCTCCAAATGTAG-3′; and forward for CTGF, 5′-TGGCCCTGACCCAACTATGA-3′; reverse for CTGF, 5′-CTTAGAACAGGCGCTCCACTCT-3′; and forward for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-TCCTGCACCACCAACTGCTTAG-3′; reverse for GAPDH, 5′-AGTGGCAGTGATGGCATGGACT-3′. To control for variations in the amount of DNA available for PCR in the different samples, the results were normalized to GAPDH mRNA levels.
Western Blot Analysis
Tissue samples of renal tissues were lysed and prepared using radioimmunoprecipitation assay (RIPA) plus buffer (Sangon Biotech). Protein concentration was measured using bicinchoninic acid (BCA) protein assay kit (Pierce Biotech Inc., Rockford, IL, USA). Protein samples were electrophoresed on sodium dodecyl sulfate (SDS)–10% polyacrylamide gel and transferred to nitrocellulose membranes, and then blocked in 5% nonfat milk for 1 h in PBS containing 0.1% Tween 20 (PBST). The nitrocellulose membrane was then incubated overnight at 4°C with monoclonal mouse anti-TGF-β1 antibody (diluted 1:2000; Abcam, Cambridge, UK) or polyclonal rabbit anti-CTGF antibody (1:3000; Abcam, Cambridge, UK). The membrane was incubated in diluted HRP-conjugated goat anti-mouse or goat anti-rabbit secondary antibody (Sangon Biotech). The protein bands were detected using the enhanced chemiluminescence system. The intensity of the Western blot bands was quantified by densitometric analysis. The results were expressed as the ratio of intensity of the protein of interest to that of β-actin.
Statistical Analysis
The results are expressed as mean ± SD. One-way analysis of variance (ANOVA) analysis followed by Tukey’s post hoc test was used to compare group means. All statistical analyses were performed using the SPSS 11.0 (SPSS Inc., Chicago, IL, USA) statistical software. p < 0.05 was considered statistically significant.
RESULTS
Effects of Quercetin on Body Weight, Kidney Weight/Body Weight, and Blood Glucose of Diabetic Rats
The values of body weight, kidney weight/body weight, and blood glucose in the three groups are presented in . Twelve weeks after STZ injection, diabetic rats exhibited increased blood glucose levels than control group. Body weights were significantly decreased and kidney weight/body weight was significantly increased in diabetic rats compared with controls. Quercetin treatment effectively reduced the increased level of kidney weight/body weight in diabetic rats. Administration of quercetin did not affect blood glucose levels and body weights in the diabetic rats.
Table 1. Metabolic and renal parameters of the three groups.
Quercetin-Induced Improvement of the Renal Function in Diabetic Rats
Elevated levels of UAE, sCr, BUN, and Ccr were found in diabetic group compared with control group. Quercetin treatment significantly decreased the elevated levels of UAE, sCr, BUN, and Ccr. The characteristics of renal function in the three groups are shown in .
Inhibition of the Overexpressions of TGF-β1 and CTGF by Quercetin
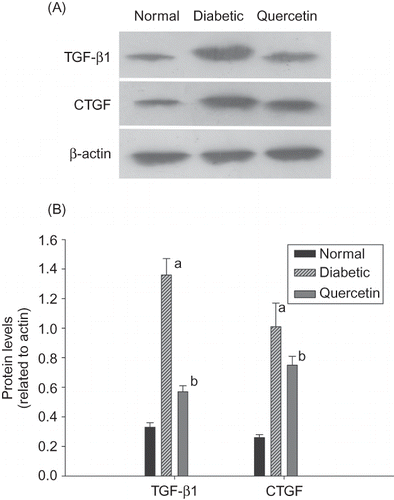
Using real-time PCR, we found that the expressions of TGF-β1 and CTGF were significantly elevated 3.6- and 2.8-fold in diabetic rats compared with controls. We also performed Western blot analysis to determine the expression levels of TGF-β1 and CTGF protein. TGF-β1 and CTGF protein expressions were dramatically increased in diabetic rats than controls (). Administration of quercetin attenuated the overexpressions of TGF-β1 and CTGF in diabetic rats ( and ).
Figure 1. Gene expressions of transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF) were quantified by real-time PCR in the renal tissues of three groups (n = 8). Data are presented as fold change of transcripts for target gene in indicated groups normalized to GAPDH.
Note: ap < 0.01 for differences versus control group; bp < 0.01 for differences versus diabetic group.
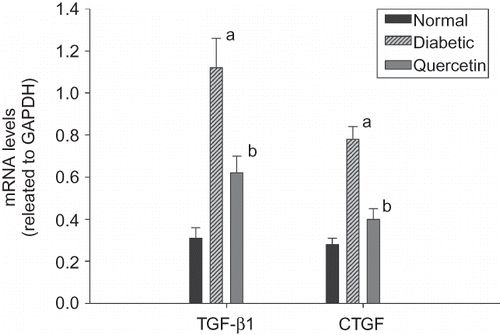
Figure 2. (A) Representative Western blots showing transforming growth factor-β1 (TGF-β1), connective tissue growth factor (CTGF), and β-actin protein levels in the renal tissues of three groups. (B) Quantification of protein levels using TGF-β1/β-actin and CTGF/β-actin was expressed as mean ± SD (n = 12 per group) in each column.
Note: ap < 0.01 compared with the values of control rats; bp < 0.01 compared with the values of untreated diabetic rats.
DISCUSSION
The results of our study revealed that quercetin treatment effectively reduced the increased level of kidney weights in diabetic rats. The elevated levels of UAE, sCr, BUN, and Ccr were also significantly improved after the administration of quercetin in diabetic rats. In addition, quercetin treatment significantly inhibited the overexpressions of TGF-β1 and CTGF in diabetic rats. As we know, it is the first study that demonstrates quercetin treatment ameliorates renal function through an inhibitory effect on the overexpressions of TGF-β1 and CTGF in diabetic rats.
Albuminuria is a clinically important marker of early DN. Proteinuria can arise from tubular cell injury by two mechanisms: release of tubular epithelial cell proteins into the urine and failure to reabsorb filtered low molecular weight proteins.Citation13 Decreased glomerular filtration rate (GFR) is the main characteristic of DN.Citation14 Quercetin was suggested to improve the elevated levels of UAE, sCr, BUN, and Ccr in diabetic rats. This is consistent with other studies. Quercetin treatment markedly attenuated the cadmium-induced biochemical alterations in serum including increased levels of serum urea, uric acid, and creatinine.Citation15 The creatinine and urea clearance were significantly improved by quercetin treatment in an experimental model of acute renal failure in rats.Citation16 These results indicate that quercetin could be substituted as a dietary supplement to prevent or treat DN.
A growing body of evidence suggests that TGF-β1, a fibrogenic cytokine, plays an important role in the development of DN.Citation17,18 Urinary TGF-β levels were higher in type 2 diabetic subjects with elevated albumin excretion rate than those with normoalbuminuria and controls.Citation19 Urinary TGF-β excretion may not only be a marker of evolving nephropathy but may be implicated in the pathogenesis of tubulointerstitial fibrosis as well as glomerulosclerosis in DN.Citation20 TGF-β1 induces the synthesis and accumulation of ECM. It is known to play a central role in the process of mesangial expansion, glomerular sclerosis, and interstitial fibrosis.Citation19 In this study, quercetin was demonstrated to have an inhibitory effect on the overexpression of TGF-β1 in diabetic rats. This indicates that quercetin may ameliorate the renal function by inhibiting TGF-β1 pathway. Similar results were also found in some studies performed in other tissues. Treatment with quercetin led to less TGF-β1-positive cells and reduced expression of TGF-β1 in bleomycin-induced pulmonary fibrosis tissues.Citation21 Furthermore, quercetin was found to effectively block the TGF-β/Smad-signaling pathway in keloid fibroblasts.Citation8
CTGF plays an important role in ECM synthesis and fibrosis. Upregulation of CTGF has been suggested to contribute to glomerular basement membrane thickening and albuminuria in DN.Citation22 TGF-β was demonstrated to upregulate the expression of CTGF and then promote the deposition of ECM components in mesangial cells.Citation7 Urinary CTGF in patients with DN was significantly higher compared with those with microalbuminuria and normoalbuminuria control subjects. Urinary CTGF was positively correlated with UAE and negatively correlated with GFR in patients with DN.Citation23 In addition, it has been reported that the downregulation of CTGF reduced proteinuria and attenuated the progression of DN in mouse models.Citation24 Our results suggested that the overexpression of CTGF was attenuated by quercetin treatment in diabetic rats. This result indicates that quercetin may reduce the increased expression of CTGF directly, or block the TGF-β pathway and then inhibit CTGF expression indirectly. Our results were consistent with another study, in which quercetin treatment was found to decrease the increased expression of CTGF mRNA in hepatic stellate cells.Citation11
The potential limitations of these data merit consideration. First, our study only demonstrated that quercetin, the most common of the flavonoid aglycones, had beneficial effects on DN. Further study might be performed to investigate whether other kinds of flavonoid aglycones contained in the diet have similar effects. Moreover, the results are still preliminary and not directly related to humans.
In summary, we found that experimental diabetic rats were accompanied by impaired renal function and an associated overexpression of TGF-β1 and CTGF. Increased TGF-β1 and CTGF mRNA and protein expression were both ameliorated by treatment of diabetic rats with quercetin. Further preclinical research into the utility of quercetin treatment may indicate its usefulness as a valuable therapeutic approach in DN.
ACKNOWLEDGMENT
We thank the staff of the Laboratory of Metabolism Disease and Research Center of Molecular Medicine, Fujian Medical University for their excellent technical assistance.
Declaration of interest: We certify that all authors have no financial or other conflict of interests in connection with this submitted article.
REFERENCES
- Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23:B21–B29.
- Brenner BM, Cooper ME, de Zeeuw D, . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869.
- Balakumar P, Arora MK, Ganti SS, Reddy J, Singh M. Recent advances in pharmacotherapy for diabetic nephropathy: Current perspectives and future directions. Pharmacol Res. 2009;60:24–32.
- Paczek L, Gaciong Z, Bartlomiejczyk I, Sebekova K, Birkenmeier G, Heidland A. Protease administration decreases enhanced transforming growth factor-beta 1 content in isolated glomeruli of diabetic rats. Drugs Exp Clin Res. 2001;27:141–149.
- Han DC, Hoffman BB, Hong SW, Guo J, Ziyadeh FN. Therapy with antisense TGF-beta1 oligodeoxynucleotides reduces kidney weight and matrix mRNAs in diabetic mice. Am J Physiol Renal Physiol. 2000;278:F628–F634.
- Umezono T, Toyoda M, Kato M, . Glomerular expression of CTGF, TGF-beta 1 and type IV collagen in diabetic nephropathy. J Nephrol. 2006;19:751–757.
- Connolly SB, Sadlier D, Kieran NE, Doran P, Brady HR. Transcriptome profiling and the pathogenesis of diabetic complications. J Am Soc Nephrol. 2003;14:S279–S283.
- Phan TT, Lim IJ, Chan SY, Tan EK, Lee ST, Longaker MT. Suppression of transforming growth factor beta/smad signaling in keloid-derived fibroblasts by quercetin: Implications for the treatment of excessive scars. J Trauma. 2004;57:1032–1037.
- Kanter M. Protective effect of quercetin on liver damage induced by biliary obstruction in rats. J Mol Histol. 2010;41:395–402.
- Huang BF, Wang W, Fu YC, Zhou XH, Wang X. The effect of quercetin on neointima formation in a rat artery balloon injury model. Pathol Res Pract. 2009;205:515–523.
- Mao YQ, Liu XJ, Jiang Y, Wu HB. Effect of quercetin on the signal pathway of TGFbeta1 in activated hepatic stellate cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35:802–805.
- Anjaneyulu M, Chopra K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2004;31:244–248.
- Barratt J, Topham P. Urine proteomics: The present and future of measuring urinary protein components in disease. CMAJ. 2007;177:361–368.
- Rosolowsky ET, Niewczas MA, Ficociello LH, Perkins BA, Warram JH, Krolewski AS. Between hyperfiltration and impairment: Demystifying early renal functional changes in diabetic nephropathy. Diabetes Res Clin Pract. 2008;82:S46–S53.
- Renugadevi J, Prabu SM. Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol. 2010;62:471–481.
- Chander V, Singh D, Chopra K. Reversal of experimental myoglobinuric acute renal failure in rats by quercetin, a bioflavonoid. Pharmacology 2005;73:49–56.
- Jeong HS, Park KK, Park KK, . Effect of antisense TGF-beta1 oligodeoxynucleotides in streptozotocin-induced diabetic rat kidney. J Korean Med Sci. 2004;19:374–383.
- Ziyadeh FN. Mediators of diabetic renal disease: The case for tgf-Beta as the major mediator. J Am Soc Nephrol. 2004;15:S55–S57.
- Ha SW, Kim HJ, Bae JS, . Elevation of urinary betaig-h3, transforming growth factor-beta-induced protein in patients with type 2 diabetes and nephropathy. Diabetes Res Clin Pract. 2004;65:167–173.
- Wang SN, LaPage J, Hirschberg R. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int. 2000;57:1002–1014.
- Baowen Q, Yulin Z, Xin W, . A further investigation concerning correlation between anti-fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. Eur J Pharmacol. 2010;642:134–139.
- Mason RM. Connective tissue growth factor (CCN2), a pathogenic factor in diabetic nephropathy. What does it do? How does it do it? J Cell Commun Signal. 2009;3:95–104.
- Nguyen TQ, Tarnow L, Andersen S, . Urinary connective tissue growth factor excretion correlates with clinical markers of renal disease in a large population of type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29:83–88.
- Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007;21:3355–3368.
