Abstract
Objective: Apoptosis and its regulatory mechanisms take part in renal ischemia–reperfusion (I/R) injury which can result in acute renal failure and the inhibition of the caspase is considered as a new therapeutic strategy. In this context, we investigated the antiapoptotic and cytoprotective effects of iloprost, a prostacyclin analog, in kidney as a distant organ. Methods: Wistar albino rats were randomized into five groups (n = 12 in each) as sham, ischemia, I/R, iloprost (10 μg kg−1), and I/R + iloprost (10 μg kg−1). A 4 h reperfusion procedure was carried out after 4 h of ischemia. Caspase-8 was evaluated for death receptor-induced pathways, whereas caspase-9 was evaluated for mitochondria-dependent pathways and caspase-3 was investigated for overall apoptosis. Superoxide dismutase (SOD) enzyme activity and nitrite content as an indicator of nitric oxide (NO) production were also analyzed in kidney tissues. Results: Caspases-3, -8, and -9 were all significantly elevated in both ischemia and I/R groups compared to the sham group; however, treatment with iloprost reduced caspases-3, -8, and -9. SOD enzyme activity was attenuated by iloprost when compared to ischemic rats. The different effects of NO were found which change according to the present situation in ischemia, I/R, and treatment with iloprost. Conclusions: These findings suggested that iloprost prevents apoptosis in both receptor-induced and mitochondria-dependent pathways in renal I/R injury and it may be considered as a cytoprotective agent for apoptosis. Understanding the efficiency of iloprost on the pathways for cell death may lead to an opportunity in the therapeutic approach for renal I/R injury.
INTRODUCTION
Numerous vascular and muscular traumas such as thrombosis and embolism, even surgeries and diseases in the extremities may cause ischemia–reperfusion (I/R) injury in skeletal muscles.Citation1 Skeletal muscles have a higher endurance to ischemia than most of the other organs. But prolonged I/R injury is not just limited to the limbs; it may also lead to the development of systemic inflammatory response syndrome, multiple-organ dysfunction syndrome,Citation2,3 and renal dysfunction.Citation4 Renal ischemia caused during surgical procedures, due to the presence of shock, and during transplantation can result in acute renal failure.Citation4,5
While ischemia prepares the tissues to be ready for reperfusion damage, it consequently causes early and irreversible tubular injury in the kidneys.Citation4 Although reperfusion is essential for the viability and recovery of the kidneys, ischemia followed by reperfusion initiates a complex cascade of events such as generation of free radicals and secretion of chemoattractants.Citation3,5
I/R injury was known to be caused particularly by necrosis; however, over the past decade it was pointed out that cell death, called apoptosis, also plays an important role in renal I/R injury.Citation6–8 It has been established that morphological and biochemical hallmarks of tubular apoptotic cell death have been observed in renal I/R injury.Citation9,10 The oligonucleosome-length DNA fragmentation (approximately 200 bp) which has been remarked as one of the biochemical markers and other molecular evidence of apoptosis were defined in renal I/R injury.Citation11–13
Caspase activation has been implicated in the development of renal I/R injury.Citation14–17 Caspases are a most extensively studied group of cysteine proteases that lead to apoptosis. Most caspases are expressed as procaspases, which refer to their inactive form. Activation of one activates others and leads to the commencement of the proteolytic breakdown. Although the members of the caspase family are numerous, caspases-3, -8, and 9 are the most important members of this group. Caspase-3 is the “executioner” or “effector” caspase which is widely found as the provoking agent in apoptosis. There are two distinct signaling pathways in apoptosis: receptor-dependent (extrinsic) and mitochondrial-dependent (intrinsic) pathways. Caspase-9 is the initiator caspase of the mitochondrial pathway whereas caspase-8 is the initiator caspase of death receptor-induced apoptosis.Citation18–20
Iloprost is a stable carbocyclic analog of prostacyclin (PGI2) which is commonly used in the treatment of arterial occlusive disorders, Buerger’s disease, heart failure, ischemic heart disease, peripheral vascular disorders, pulmonary arterial hypertension, and Raynoud’s disease.Citation21 Iloprost mimics the effects of physiological PGI2 that can be summarized as vasodilatation, decreasing the inflammatory reactions, and inhibition of both platelet aggregation and leukocyte activation.Citation22,23
Since iloprost was investigated and some evident results were found about its cytoprotective effects, reduction of inflammatory reactions and prevention of reactive oxygen species (ROS) generation in many disorders,Citation21–23 we aimed to elucidate its antiapoptotic effects by evaluating caspases-3, -8, and -9 and antioxidant capacity as well as its effect on nitric oxide (NO) in renal I/R injury as a distant organ.
MATERIALS AND METHODS
Animals
Healthy male Wistar albino rats, 5–6 weeks old (n = 60) and weighing 180–250 g, were purchased from the Animal Research Laboratory of our institution. Rats were subjected to this study after being acclimatized to the laboratory conditions for a week before any applications were initiated. They were housed in stainless steel cages in an acclimated room at a constant temperature of 25°C and relative humidity of 55 ± 8% with the realization of 12 h light–dark cycle. They were fed on a standard diet with ad libitum access to drinking water. All experiments were performed strictly in accordance with the Principles of Laboratory Animal Care (NIH publication no. 85-23, revised 1985).
Experimental Design
Rats were randomized into five groups as sham, ischemia, I/R, iloprost (10 μg kg−1; Ilomedin 20, Schering, Germany), and I/R + iloprost (10 μg kg−1). The number of rats in each group was 12. After the application of anesthesia with intramuscular 100 μg kg−1 ketamine, both hind limbs were occluded with the application of tourniquets. A 4 h of ischemia was carried out in the ischemia group. A 4 h reperfusion procedure was carried out after 4 h of ischemia by removing the tourniquets in the I/R group. Iloprost (10 μg kg−1) was administered to the rats in the iloprost group in 1 mL of saline from the tail veins. Rats in the I/R + iloprost group received 10 μg kg−1 iloprost in the same way 10 min before the removal of the tourniquets. Rats in the sham group received an equal amount of 0.9% NaCl solution via the same route and over the same period of time. At the end of the experimental protocol, the rats were killed and their right kidneys were dissected and harvested.
Preparation of Kidney Homogenates
Kidneys were homogenized in lysis buffer and centrifuged at 14,000 × g for 10 min at 4°C. The cytosolic extract was removed gently. Superoxide dismutase (SOD) enzyme activity and nitrite levels were determined in the supernatants. Protein levels were evaluated according to the method used by Lowry et al.Citation24 Caspases-3, -8, and -9 were determined according to the manufacturer’s instructions.
Determination of Caspase-3
Activity of caspase-3 was evaluated by the Caspase-3/CPP32 Colorimetric Assay Kit (BioVision Research Product, Mountain View, CA, USA). Enzymatic activity of caspase-3 was determined according to the manufacturer’s instructions. For each assay, 50 μg protein was diluted up to 50 μL with cell lysis buffer. This assay kit was based on spectrophotometric method for detecting chromophore p-nitroaniline (pNA) after cleavage from the labeled substrate DEVD-pNA. pNA light emission was quantified using a microplate reader at 405 nm. Caspase-3 activity was measured by quantitative analysis of the pNA released from DEVD-pNA.
Determination of Caspase-8
Activity of caspase-8 was evaluated by the FLICE/Caspase-8 Colorimetric Assay Kit (BioVision Research Product). Enzymatic activity of caspase-8 was determined according to the manufacturer’s instructions. For each assay, 50 μg protein was diluted up to 50 μL with cell lysis buffer. This assay kit was based on spectrophotometric method for detecting chromophore pNA after cleavage from the labeled substrate IETD-pNA. pNA light emission was quantified using a microplate reader at 405 nm. Caspase-8 activity was measured by quantitative analysis of the pNA released from IETD-pNA.
Determination of Caspase-9
Activity of caspase-9 was evaluated by the Caspase-9 Colorimetric Assay Kit (BioVision Research Product). Enzymatic activity of caspase-9 was determined according to the manufacturer’s instructions. For each assay, 50 μg protein was diluted up to 50 μL with cell lysis buffer. This assay kit was based on spectrophotometric method for detecting chromophore pNA after cleavage from the labeled substrate LEHD-pNA. pNA light emission was quantified using a microplate reader at 405 nm. Caspase-9 activity was measured by quantitative analysis of the pNA released from LEHD-pNA.
Measurement of SOD
The SOD enzyme activity was assayed according to the method of McCord et al.Citation25 The principle of the method depends on xanthine and xanthine oxidase to generate superoxide radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride to form a red formazan dye. The SOD activity is evaluated by the degree of inhibition of this reaction. The optical density of this substance was measured at 505 nm. The results were stated in units per milligram of protein.
Measurement of Nitrite Levels
The concentrations of nitrite in tissue homogenates were measured by the diazotization method based on the Griess reaction, which is an indirect assay for NO synthase (NOS) activity.Citation26 Briefly, samples (50 μL) were pipetted into 96-well microtiter plates and an equal volume of Griess reagent (1% sulfanylamide (25 μL) and 0.1% N-1-naphthylethylenediamine dihydrochloride (25 μL in 2.5% orthophosphoric acid) was added to each well. After incubation for 15 min at room temperature, absorbance was measured at 550 nm with a microplate reader. Linear regression analysis was used to calculate the nitrite concentrations in the tissue homogenates from the standard calibration curves of sodium nitrite. The results were stated as μM per milligram of protein.
Statistical Analysis
Shapiro–Wilk test was used to determine whether all parameters were normally distributed and it was found that all parameters were normally distributed. Descriptive statistics (mean ± standard deviation) were calculated in each group for all parameters. Variance analyses were used to test the differences between the groups for each parameter. One-way ANOVA was used for parameters to provide homogeneity of variance; Kruskall–Wallis and Welch tests were used for parameters to provide heterogeneity of variance. Kruskall–Wallis test was used for the parameters of caspases-3, -8, and -9 and Welch test was used for the SOD parameter. Student–Newman–Keuls, Duncan, and Games–Howell tests were used for multiple comparisons. Data were analyzed using SPSS v11.5 (SPSS Inc., Chicago, IL, USA) and MedCalc v11.2.1 (MedCalc software, Mariakerke, Belgium) statistical packet programs and graph was obtained in STATISTICA v8.0 (Statsoft Inc., Tulsa, OK, USA) packet program. The results were considered statistically significant if p-values were less than 0.05.
RESULTS
Effects of Iloprost on Caspase-3, -8, and -9 Enzyme Activities in Renal I/R Injury
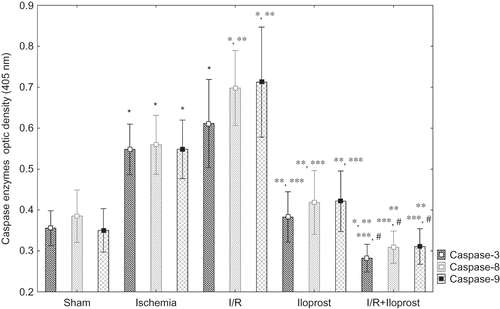
In this study, we evaluated caspase-3, -8, and -9 enzyme activities as apoptotic markers. Renal caspase-3, -8, and -9 enzyme activities were all increased significantly in both ischemia and I/R groups when compared with the sham (p < 0.05) (). The elevation in caspase-3, -8, and -9 enzyme activities was reduced by iloprost. Iloprost (10 μg kg−1) treatment to the I/R + iloprost subjects decreased the caspase-3, -8, and -9 enzyme activities when compared with both the ischemia and the I/R groups. Even more, iloprost (10 μg kg–1) treatment to I/R + iloprost subjects decreased the caspase-3 enzyme activity in comparison with the sham group. Administration of iloprost (10 μg kg−1) to healthy rats did not have any effects. Caspase-3, -8, and -9 enzyme activities were nearly the same in sham and iloprost groups. The overall results are demonstrated in .
Figure 1. Effects of iloprost on caspases-3, -8, and -9 in kidney as a distant organ. Treatment of iloprost in I/R + iloprost subjects decreased the caspase-3, -8, and -9 enzyme activities as compared with both the ischemia and the I/R groups. Even more, iloprost (10 μg kg–1) treatment in I/R + iloprost subjects decreased the caspase-3 enzyme activity as compared with the sham group. Values are expressed as means ± SEM.
Note: p < 0.05 was considered to be significant. *Significantly different from sham, **significantly different from ischemia, ***significantly different from I/R, and #significantly different from iloprost.
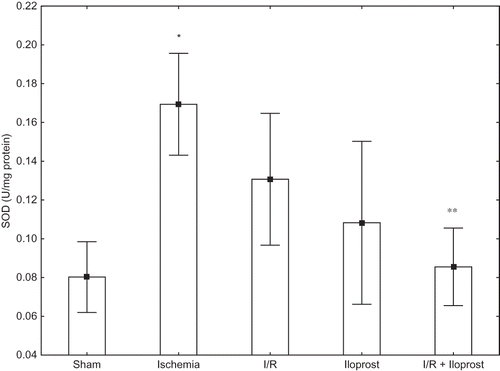
Effects of Iloprost on SOD Enzyme Activity in Renal I/R Injury
Although SOD enzyme activities were altered in both ischemia (0.080 ± 0.026 vs. 0.169 ± 0.039) and I/R groups (0.080 ± 0.026 vs. 0.131 ± 0.044), the increase was only statistically significant in the ischemic group when compared with the sham group (p < 0.005). SOD enzyme activity was higher in rats subjected to ischemia alone rather than I/R. Administration of iloprost (10 μg kg−1) to the rats that constituted the I/R + iloprost group caused a decrease in the SOD enzyme activity in comparison with the ischemic rats’ group ().
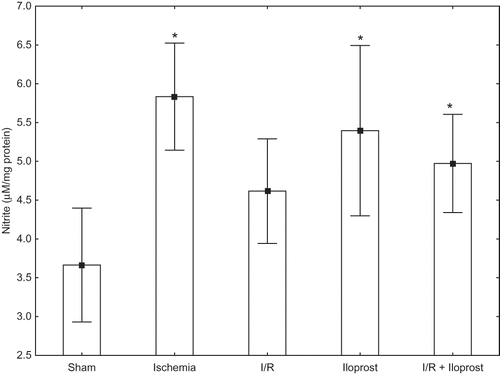
Effects of Iloprost on NO in Renal I/R Injury
Similar to SOD enzyme activity, renal nitrite levels were also altered in both ischemia (3.665 ± 1.025 vs. 5.834 ± 1.087) and I/R groups (3.665 ± 1.025 vs. 4.616 ± 1.061); the increase was only statistically significant in the ischemia group when compared with the sham group (p < 0.005). However, administration of iloprost (10 μg kg−1) to the rats that constituted the iloprost and I/R + iloprost groups increased the nitrite levels. Iloprost caused an increase in the levels of nitrite in the iloprost and I/R + iloprost groups ().
DISCUSSION
The results of the present study suggest that apoptosis was induced during renal I/R injury. The cytoprotective effect of iloprost was observed by reduced apoptosis by blocked proteolytic breakdown of the caspases in the kidneys. As a secondary finding, this study also indicated the differential effects of NO in renal I/R injury.
Several evident reports have published that apoptosis and its regulatory mechanisms take part in acute renal failureCitation27,28 and inhibition of the caspases is considered as a new therapeutic strategy in renal I/R injury.Citation16,29–33 Therefore, we investigated the antiapoptotic effects of iloprost in renal injury as a distant organ by evaluating caspase-8 for death receptor-induced apoptosis, caspase-9 for mitochondria-dependent apoptosis, and caspase-3 for overall apoptosis. We found that renal caspase-3, -8, and -9 enzyme activities were increased significantly both in ischemia and in I/R groups when compared with the sham. There is an increasing evidence that hypoxic stress induces apoptosis in the renal proximal tubular cells via both receptor-induced and mitochondria-dependent pathways.Citation17,34 Induction of proapoptotic mechanisms were reported during prolonged ischemia such as enhancing the Bax/Bcl-2 ratio, caspase-3 expression, and poly-(ADP-ribose)-polymerase fragments resulting with DNA fragmentation and augmentation of apoptotic cells in renal proximal and distal tubules.Citation35 ShiCitation33 also reported the enhancement of caspase-3 activity in renal I/R injury.
PGI2 is found to protect tubular cells from gentamicin-induced apoptosis via peroxisome proliferator-activated receptor alpha-signaling pathwayCitation36 and also prevents pulmonary endothelial cell apoptosis which is induced by cigarette smoking.Citation37 In the present study, increased caspase-3, -8, and -9 enzyme activities were significantly reduced by iloprost, a stable PGI2 analog, when compared with both ischemia and I/R groups. Moreover, iloprost treatment decreased the caspase-3 enzyme activity in I/R + iloprost group when compared with the sham group.
It has been reported that iloprost did not augment monocyte apoptosis, TNF-alpha production, and TNF-alpha receptor II in the critical limb ischemia patients.Citation38 Injury in cardiomyocytes induced by H2O2 and doxorubicin was also reduced by iloprost via the induction of cyclooxygenase-2, which is mediated by extracellular signal-regulated kinases 1/2 (ERK1/2).Citation39 In addition, it was also mentioned that iloprost induces apoptosis of vascular smooth muscle cells via a cAMP-mediated inhibition of ERK activity.Citation40
The pathophysiological process involved in reperfusion injury is a complex cascade which also includes release of ROS that contribute to the ischemic acute renal failure.Citation5 ROS have been observed in several experimentally induced I/R injury in different organs such as heart, liver, brain, and kidney and it has been established that ROS have a crucial role in reperfusion injury.Citation3–5,41 It was revealed that oxidative stress contributes to I/R-induced renal apoptosis.Citation42,43 Furthermore, it was also established that ROS activate multiple steps of receptor-induced and mitochondria-dependent pathways of apoptosis.Citation28
In this study, the ROS generation in response to renal I/R injury was evaluated by SOD enzyme activity. Although SOD enzyme activities were altered in both ischemia and I/R groups, the increase was only statistically significant in the ischemic group when compared with the sham group. Surprisingly, SOD enzyme activity was higher in rats subjected to ischemia than the rats in I/R. It is well known that the rapid entry of molecular oxygen into the cell during reperfusion period provokes the ROS generation more than the ischemia period.Citation44 This may be explained by the production of peroxynitrite in I/R injury under oxidative stress.Citation45 It has been reported that NO reacts rapidly with superoxide, almost in diffusion-controlled rates [(6.7 ± 0.9) × 109 L mol–1 s–1], that results in producing peroxynitrite which is faster than the removal of superoxide by SOD.Citation46 As this reaction is faster than the reaction rate for superoxide with SOD, this may clarify the reduction of superoxide radicals. The depletion of superoxide in the I/R group should be the result of interaction between NO and superoxide.
The increased SOD enzyme activity is considered as a hallmark of free-radical generation in ischemia and I/R processes. Iloprost possibly led to the decrease of SOD enzyme activity by scavenging the free-oxygen radicals in the kidneys when compared with the ischemic rats. It was reported that iloprost prevents I/R injury by reducing myeloperoxidase and malondialdehyde and protection of total antioxidant capacity in kidney as a distant organ.Citation47 Similar results were reported by Ozcan et al.Citation48 Iloprost also exerts its antioxidant efficiency in I/R injury of skeletal musclesCitation49 and lungs.Citation50
It is well known that NO is a multifunctional mediator which involves in both physiological and pathological processes in renal I/R injury.Citation45 In renal cells, NO is synthesized from the terminal guanido nitrogen atom of l-arginine by NOS which has three isoforms: inducible NOS (iNOS),Citation51 endothelial NOS (eNOS),Citation52 and neuronal NOS.Citation53 iNOS is expressed following the induction of inflammatory mediators and cytokines and catalyzes large amounts of harmful NO whereas eNOS is considered the protective isoenzyme and produces low concentration of NO which is beneficial for the endothelial functions and integrity.Citation54
In our study, nitrite levels were also altered in both ischemia and I/R groups, similar to the SOD enzyme activity, but the increase was only statistically significant in the ischemic group when compared with the sham group. This result supported the interaction between NO and superoxide as described above. But, on the other hand, increased nitrite levels were also observed by the administration of iloprost to the rats that constituted the iloprost and the I/R + iloprost groups. As a result, iloprost increased the nitrite levels in iloprost and the I/R + iloprost groups.
There are many distinct reports about the interaction between NO and prostaglandins.Citation55–57 It was indicated that PGI2 plays a substantial role in vascular functions and regulation of intracellular concentration of cyclic AMP. It has been reported that iloprost stimulates NO formation in the rat aorta and it was suggested that adenosine activates endothelial A1 receptors during systemic hypoxia and it further causes synthesis of prostaglandins, thereby the activation of cyclic AMP which results in NO generation and muscle vasodilatation.Citation58
Although nitrite is considered as one of the toxic metabolites in pathological conditions, protective effects of nitrite has been also demonstrated in kidneys.Citation59 Even Elrod et al.Citation60 demonstrated that NO derived from eNOS is transported in the blood, metabolized in distant organs, and exerts cytoprotective effects in I/R injury.
NO also has a crucial role as a mediator of apoptosis in renal I/R injury. It was demonstrated that NO, iNOS, and eNOS were related with the development of apoptosis during renal I/R via eNOS overexpression and NO production and also expression/activation of the iNOS/NO system induced by eNOS.Citation61
In summary, we present the novel finding that iloprost prevents apoptosis both by receptor-induced and mitochondria-dependent pathways. The present study not only demonstrated the effects of iloprost on extrinsic and intrinsic pathways of apoptosis but also exerted the importance of caspases-3, -8, and -9 in renal I/R injury. At the same time, we found out different effects of NO which change according to the present situation during renal ischemia and I/R injury. Besides, we demonstrated the association between iloprost and NO in renal I/R injury. Understanding the efficiency of iloprost on the pathways for cell death may have an opportunity in the therapeutic approach to renal I/R injury.
ACKNOWLEDGMENT
This research was supported by the Research Foundation of Mersin University, Mersin, Turkey (Grant no. BAP-ECZ F TEB (NC) 2007).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Wang WZ, Fang XH, Stephenson LL, Khiabani KT, Zamboni WA. Ischemia/reperfusion-induced necrosis and apoptosis in the cells isolated from rat skeletal muscle. J Orthop Res. 2008;26(3):351–356.
- Neary P, Redmond HP. Ischemia-Reperfusion Injury and the Systemic Inflammatory Response Syndrome. Oxford: Blackwell Science; 1999:123–136.
- Carden DL, Granger DN. Pathophysiology of ischemia-reperfusion injury. J Pathol. 2000;190:255–266.
- Weight SC, Bell F, Nicholson ML. Renal ischemia-reperfusion injury. Br J Surg. 1996;83:162–170.
- Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43(5):1160–1178.
- Lieberthal W, Koh JS, Levine JS. Necrosis and apoptosis in acute renal failure. Semin Nephrol. 1998;18:505–518.
- Saikumar P, Venkatachalam MA. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol. 2003;23:511–521.
- Castaneda P, Swiatecka-Urban A, Mitsnefes MM. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemia reperfusion injury. Transplantation 2003;76:50–54.
- Qiao X, Chen X, Wu D, . Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci. 2005;60:830–839.
- Burns AT, Davies DR, McLaren AJ, Cerundolo L, Morris PJ, Fuggle SV. Apoptosis in ischemia/reperfusion injury of human renal allografts. Transplantation 1998;66:872–876.
- Nogae S, Koji T, Nakanishi Y. Induction of apoptosis in ischemia-reperfusion kidney model: Appearance of DNA strand breaks and expression of FAS mRNA. J Am Soc Nephrol. 1994;5:905.
- Schumer M, Colombel MC, Sawczuk IS, . Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992;140:831–838.
- Beeri R, Symon Z, Brezıs M. Rapid DNA fragmentation from hypoxia along the thick ascending limb of rat kidneys. Kidney Int. 1995;47:1806–1810.
- Wolfs TG, de Vries B, Walter SJ, . Apoptotic cell death is initiated during normothermic ischemia in human kidneys. Am J Transplant. 2005;5(1):68–75.
- Jani A, Ljubanovic D, Faubel S, Kim J, Mischak R, Edelstein CL. Caspase inhibition prevents the increase in caspase-3, -2, -8 and -9 activity and apoptosis in the cold ischemic mouse kidney. Am J Transplant. 2004;4(8):1246–1254.
- Edelstein CL, Shi Y, Schrıer RW. Role of caspases in hypoxia-induced necrosis of rat renal proximal tubules. J Am Soc Nephrol. 1999;10:1940–1949.
- Kaushal GP, Basnakian AG, Shah SV. Apoptotic pathways in ischemic acute renal failure. Kidney Int. 2004;66(2):500–506.
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290.
- Salvesen GS, Dixit VM. Caspases: Intracellular signaling by proteolysis. Cell 1997;91:443–446.
- Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29–55.
- Mubarak KK. A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Resp Med. 2010;104:9–21.
- Granger DN, Kubes P. The microcirculation and inflammation: Modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55:662–675.
- Grant SM, Goa KL. Iloprost: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischemia and extracorporeal circulation procedures. Drugs 1992;43:889–924.
- Lowry OH, Rosebrough NJ, Farr AL, Randall JR. Protein measurement with the folin phenol reagent. J Biol Chem. 1961;193(1):265–275.
- McCord JM, Fridovıch I. Superoxide dismutase. An enzymatic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6049–6055.
- Tunctan B, Korkmaz B, Yildirim H, Tamer L, Atik U, Buharalioglu CK. Increased production of nitric oxide contributes to renal oxidative stress in endotoxemic rat. Am J Infect Dis. 2005;1(2):111–115.
- Ortiz A, Justo P, Sanz A, Lorz C, Egido J. Targeting apoptosis in acute tubular injury. Biochem Pharmacol. 2003;66(8):1589–1594.
- Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80(1):29–40.
- Chatterjee PK, Todorovic Z, Sivarajah A, . Differential effects of caspase inhibitors on the renal dysfunction and injury caused by ischemia-reperfusion of the rat kidney. Eur J Pharmacol. 2004;503:173–183.
- Daemen MA, Veer VC, Denecker G, . Inhibition of apoptosis induced by ischemia–reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549.
- Daemen MA, de Vries B, Van’t Veer C, Wolfs TG, Buurman WA. Apoptosis and chemokine induction after renal ischemia-reperfusion. Transplantation 2001;71(7):1007–1011.
- Yang B, Johnson TS, Haylor JL, . Effects of caspase inhibition on the progression of experimental glomerulonephritis. Kidney Int. 2003;63:2050–2064.
- Shi Y, Melnikov VY, Schrierrw RW, Edelsteın CL. Downregulation of the calpain inhibitor protein calpastatin by caspases during renal ischemia-reperfusion. Am J Physiol Renal Physiol. 2000;279:509–517.
- Terada Y, Inoshita S, Kuwana H, . Important role of apoptosis signal-regulating kinase 1 in ischemic acute kidney injury. Biochem Biophys Res Commun. 2007;364(4):1043–1049.
- Chien CT, Lee PH, Chen CF. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J Am Soc Nephrol. 2001;12:973–982.
- Hsu YH, Chen CH, Hou CC, . Prostacyclin protects renal tubular cells from gentamicin-induced apoptosis via a PPARalpha-dependent pathway. Kidney Int. 2008;73:578–587.
- Nana-Sinkam SP, Lee JD, Sotto-Santiago S, . Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med. 2007;175:676–685.
- Di Renzo M, Pieragalli D, Meini S, . Iloprost treatment reduces TNF-alpha production and TNF-RII expression in critical limb ischemia patients without affecting IL6. Prostag Leukotr Ess. 2005;73(5):405–410.
- Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274(8):5038–5046.
- Li RC, Cindrova-Davies T, Skepper JN, Sellers LA. Prostacyclin induces apoptosis of vascular smooth muscle cells by a cAMP-mediated inhibition of extracellular signal-regulated kinase activity and can counteract the mitogenic activity of endothelin-1 or basic fibroblast growth factor. Circ Res. 2004;94(6):759–767.
- Mccord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163.
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14(8):2199–2210.
- Lameire NH, Vanholder R. Pathophysiology of ischemic acute renal failure. Best Pract Res Clin Anaesthesiol. 2004;18(1):21–36.
- Zimmerman BJ, Granger DN. Mechanism of reperfusion injury. Am J Med Sci. 1994;307(4):284–292.
- Pacher P, Beckman JS, Lıaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424.
- Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun. 1993;18(4):195–199.
- Aytacoglu BN, Sucu N, Tamer L, . Iloprost for the attenuation of ischemia/reperfusion injury in a distant organ. Cell Biochem Funct. 2006;24(4):341–346.
- Ozcan AV, Sacar M, Aybek H, . The effects of iloprost and vitamin C on kidney as a remote organ after ischemia/reperfusion of lower extremities. J Surg Res. 2007;140(1):20–26.
- Bozkurt AK. Alpha-tocopheral (vitamin E) and iloprost attenuate reperfusion injury in skeletal muscle ischemia/reperfusion injury. J Cardiovasc Surg. 2002;43:693–696.
- Yasa H, Yakut N, Emrecan B, . Protective effects of levosimendan and iloprost on lung injury induced by limb ischemia-reperfusion: A rabbit model. J Surg Res. 2008;147(1):138–142.
- Mattson DL, Wu F. Nitric oxide synthase activity and isoforms in rat renal vasculature. Hypertension 2000;35:337–341.
- Kone BC. Nitric oxide in renal health and disease. Am J Kidney Dis. 1997;30:311–333.
- Roczniak A, Levine DZ, Burns KD. Localization of protein inhibitor of neuronal nitric oxide synthase in rat kidney. Am J Physiol. 2000;278:702–707.
- Albrecht EW, Stegeman CA, Heeringa P, Henning RH, van Goor H. Protective role of endothelial nitric oxide synthase. J Pathol. 2003;199(1):8–17.
- Goodwin DC, Landino LM, Marnett LJ. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxide synthase and prostaglandin biosynthesis. FASEB J. 1999;13(10):1121–1136.
- Tokuyama H, Hayashi K, Matsuda H, . Role of nitric oxide and prostaglandin E2 in acute renal hypoperfusion. Nephrology 2003;8(2):65–71.
- Rajapakse NW, Flower RL, Eppel GA, Denton KM, Malpas SC, Evans RG. Prostaglandins and nitric oxide in regional kidney blood flow responses to renal nerve stimulation. Pflugers Arch. 2004;449(2):143–149.
- Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: In vivo and in vitro studies. J Physiol. 2002;544:195–209.
- Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia–reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–338.
- Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: Evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci. 2008;105(32):11430–11435.
- Viñas JL, Sola A, Genescà M, Alfaro V, Pí F, Hotter G. NO and NOS isoforms in the development of apoptosis in renal ischemia/reperfusion. Free Radic Biol Med. 2006;40(6):992–1003.