Abstract
Rationale/objectives: Data are limited regarding the use of paricalcitol in calcitriol-resistant patients with secondary hyperparathyroidism (SHPT). We aimed to evaluate the effects of paricalcitol in calcitriol-resistant hemodialysis patients with SHPT. Methods: This is a 12-month, open-label, prospective study. Forty patients with calcitriol-resistant and/or calcitriol-intolerant SHPT were included. After a washout period, all patients converted to paricalcitol with a 1:3 conversion ratio. Serum calcium and phosphorus were monitored monthly, while serum intact parathyroid hormone (iPTH) once in every 3 months. Paricalcitol dose was reduced or discontinued in case of hypercalcemia and/or hyperphosphatemia. Pre- and posttreatment electrolyte and iPTH values were compared with Student’s t-test and Wilcoxon signed-rank test, respectively. Main findings: Forty patients completed the study. Mean initiation dose of paricalcitol was 23 ± 7 μg/week. Mean serum calcium was 8.9 ± 0.8 mg/dL at baseline and 9.4 ± 0.7 mg/dL at study end (p = 0.07). Mean monthly serum phosphorus levels stayed stable. Paricalcitol was effective in reducing iPTH levels when compared with pretreatment values (747.9 ± 497.2 pg/mL, 307.3 ± 417.1 pg/mL, respectively; p < 0.001). Thirty-two patients had to discontinue intravenous (IV) paricalcitol at some time during their treatment. Main reasons for discontinuation were as follows: hyperphosphatemia (58%), hypercalcemia (25%), and iPTH < 150 pg/mL (17%). Principle conclusions: Paricalcitol was found to be effective in reducing iPTH levels in calcitriol-resistant patients with SHPT despite relatively frequent drug discontinuation rates.
INTRODUCTION
Secondary hyperparathyroidism (SHPT) is a common occurrence in end-stage renal disease (ESRD) patients and affects approximately 75% of patients undergoing hemodialysis.Citation1 SHPT is characterized by parathyroid gland hyperplasia, increased levels of serum intact parathyroid hormone (iPTH), and resultant morbidity.Citation2 SHPT is associated with bone disease, neuromuscular disease, and soft tissue and vascular calcification and potentially with accelerated atherosclerosis in ESRD patients.Citation3
Treatment of SHPT is essentially based on dietary phosphate restriction, administration of oral phosphate binders, and vitamin D (calcitriol) replacement.Citation4 Treatment of SHPT with active vitamin D compounds is logical and generally effective.Citation5 Vitamin D activates intracytoplasmic receptors, which directly inhibit iPTH synthesis.Citation6
The parent active vitamin D molecule, calcitriol, has been successfully used for the treatment of SHPT in dialysis patients.Citation7 Despite its beneficial effects on serum iPTH levels, the use of calcitriol is limited mainly by its phosphatemic and calcemic effects especially when used concomitantly with calcium-based oral phosphate binders.Citation8 Because calcitriol stimulates vitamin D receptors that increase intestinal absorption of calcium and phosphorus, with consequent hypercalcemia and hyperphosphatemia, calcitriol potentially increases the risk of ectopic vascular calcification and cardiovascular mortality.Citation4 To overcome this untoward effect of calcitriol, newer vitamin D analogues such as paricalcitol (19-nor-1α,25(OH)2D2) have been devised. Paricalcitol has lesser calcemic and phosphatemic effects compared with calcitriol.Citation9,10 A few studies have made head-to-head comparison of paricalcitol and calcitriol in the treatment of SHPT.Citation11–14 These studies have shown superiority of paricalcitol in terms of iPTH reduction with lesser degree of hyperphosphatemia and hypercalcemia in paricalcitol-treated patients compared with calcitriol treatment. On the other hand, there is scarcity of data regarding the role of paricalcitol in patients with SHPT who cannot tolerate intravenous (IV) calcitriol treatment due to hypercalcemia and/or hyperphosphatemia and who show insufficient iPTH reduction. There are only two studies that evaluated the efficacy and tolerability of paricalcitol in patients who are intolerant or resistant to calcitriol treatment.Citation15,16 There are a lot of patients with very high serum iPTH hormone levels and/or hyperphosphatemia/hypercalcemia with calcitriol treatment. These patients pose a greater challenge for caring nephrologists than native patients with SHPT.
Hence, we aimed to investigate the effects of IV paricalcitol treatment in maintenance hemodialysis patients with SHPT who are resistant to or intolerant of (owing to hypercalcemia and hyperphosphatemia) IV calcitriol treatment.
MATERIALS AND METHODS
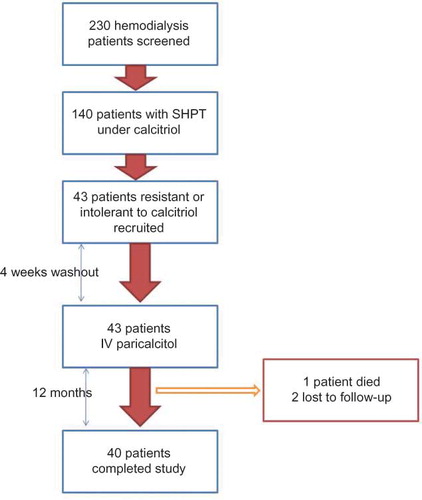
This is a 12-month, open-label, prospective intervention study conducted at two private hemodialysis centers. The flowchart of study design is depicted in . A total of 230 patients who were undergoing maintenance hemodialysis were screened. Patients with persistent SHPT for at least 6 months despite treatment with appropriate doses of IV calcitriol were recruited for the study. Inclusion criteria were as follows: duration of maintenance hemodialysis for at least 1 year, intolerance or resistance to calcitriol treatment despite appropriate dietary phosphate restriction, use of oral phosphate binders and adequate dose adjustments of calcitriol, and age >18 years. Resistance to calcitriol treatment was described as persistently elevated serum iPTH levels (>300 pg/mL) above guideline targetsCitation17 despite maximum tolerable doses of IV calcitriol treatment (up to 9 μg/week) for at least 6 months. Intolerance to calcitriol was described as necessary discontinuation of IV calcitriol for three consecutive months owing to hypercalcemia and/or hyperphosphatemia despite switching to noncalcium-containing phosphate binders and adequate dose reduction of calcitriol. The study protocol was approved by local ethics committee and all patients gave signed informed consent before enrolling in the study. The primary endpoint was the rate of decline in levels of iPTH to target levels (between 150 and 300 ng/dL) with IV paricalcitol treatment. Secondary endpoints were the frequency of hypercalcemia and/or hyperphosphatemia related to paricalcitol treatment and the frequency of treatment interruptions due to reduction of iPTH below guideline targets and hyperphosphatemia and/or hypercalcemia.
All calcitriol-resistant or calcitriol-intolerant patients were administered IV paricalcitol (Zemplar®; Abbott Laboratories, Istanbul, Turkey) with a conversion dose ratio of 1:3 (from calcitriol to paricalcitol) after a 4-week washout period. The initial paricalcitol dose was maintained for a minimum of 4 weeks and subsequent dose adjustments of paricalcitol were based on levels of serum iPTH, calcium, and phosphorus. Other medications including oral phosphate binders that patients were already receiving at the start of the study were not changed. Serum calcium and phosphorus levels along with other standard biochemical and hematologic parameters were determined at the outset of the study. iPTH (normal range: 10–69 pg/mL) was determined by chemiluminescence method (Immulite 2000; DPC, Los Angeles, CA, USA). Serum phosphorus and calcium were studied monthly while iPTH levels were studied every 3 months. For analyses, serum calcium level was corrected according to serum albumin level. In case of hyperphosphatemia and hypercalcemia, a predefined protocol was used: paricalcitol dose was reduced in half if serum phosphorus and calcium levels are found between 5.5–6 and 10.2–11 mg/dL, respectively. In case of hypercalcemia, we also switched patients to noncalcium-containing phosphate binder sevelamer. In case of elevations above these levels for calcium, phosphorus, or both, paricalcitol was discontinued; levels of calcium and phosphorus were monitored; and the drug was reinstituted after normalization of biochemical parameters. All patients underwent hemodialysis weekly thrice using a standard bicarbonate bath (calcium 1.25 mmol/L) and received proper nutritional counseling. Potential adverse effects of paricalcitol other than calcemic and phosphatemic events were also monitored every week at a respective dialysis center through comprehensive physical examination and systems review.
Statistical Analyses
All data are presented as mean ± standard deviation unless stated otherwise. Statistical differences in iPTH measurements before and after paricalcitol treatment were assessed by the Wilcoxon signed-rank test. Paired sample t-test was used to compare pre- and posttreatment values of calcium, phosphorus, and Ca × P. The data were evaluated using SPSS 17 (SPSS, Inc., Chicago, IL, USA) statistical program.
RESULTS
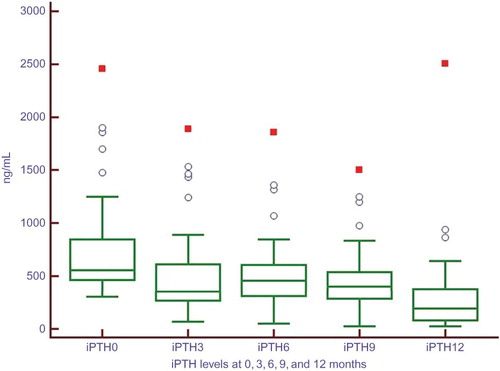
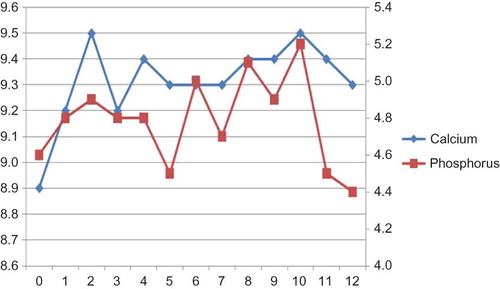
A total of 230 patients were screened to be included in the study. One hundred and forty patients (60%) have been under calcitriol treatment for SHPT. Forty-three patients (30.7% of patients using calcitriol) were deemed eligible to be included in the study. Basic demographic characteristics and laboratory data of entire study population are shown in . One patient died and two patients were lost to follow-up during the course of the study. Finally, 40 patients completed the 12-month study period and none was dropped due to adverse effects. Comorbidities of study cohort were as follows: diabetes mellitus type 2 in 8 patients (20%), hypertension in 14 (35%), and coronary artery disease in 6 (15%). Mean initiation dose of paricalcitol was 23 ± 7 μg/week; mean maintenance dose was 11 ± 4 μg/week. We did not observe any significant adverse events associated with IV paricalcitol use other than hypercalcemia and/or hyperphosphatemia. Mean serum iPTH levels showed a gradual decrease through the study period (). Mean monthly serum calcium values increased from 8.9 ± 0.8 mg/dL at baseline to 9.4 ± 0.7 mg/dL at study end. Mean monthly serum phosphorus levels stayed stable and did not exceed guideline targets () despite hyperphosphatemic surges in some patients.
Table 1. Basic demographic and laboratory characteristics of the study cohort.
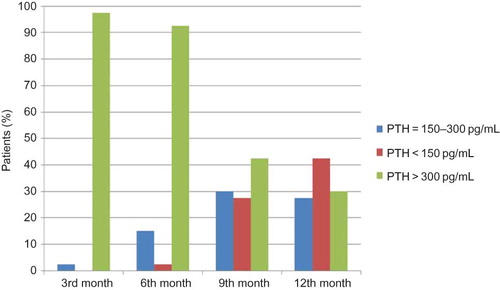
Paricalcitol was generally effective in reducing iPTH levels when compared with pretreatment values (). Thirty-nine patients (97.5%) completed the study with lower iPTH values compared with pretreatment levels. However, at the 12th month not all patients fulfilled therapeutic guideline targets. At the 12th month, 11 of 40 patients (27.5%) were at target iPTH range (). However, of all four measurements during the IV paricalcitol treatment, 17 patients (42.5%) achieved target iPTH level once, 5 patients achieved twice (12.5%), and only 1 patient achieved thrice (2.5%). Seventeen patients (42.5%) could not achieve target iPTH levels in any of the four measurements during the study period. Of the total 160 measurements of iPTH hormone, 29 measurements (18.1%) were within target limits. Totally 101 measurements (63.1%) were above 300 ng/mL.
Table 2. Comparison of pre- and posttreatment values in serum calcium, phosphorus, and iPTH levels and Ca × P.
Eight patients (20%) continued paricalcitol without any interruption throughout the study period. The remaining 32 patients (80%) had to discontinue IV paricalcitol at some time during their treatment. Median duration for paricalcitol discontinuation was 4 months (1–8 months). The majority of patients (25 of 32 patients, 78%) had to discontinue their drug more than once during the 12-month period. Median number of discontinuation was 2 (range 1–5 times). Main reasons for discontinuation of the paricalcitol treatment were as follows: hyperphosphatemia [58% of all discontinuation events, reason for discontinuation in 16 patients (40%)], hypercalcemia [25% of all discontinuation events, reason for discontinuation in six patients (15%)], and iPTH < 150 pg/mL [17% of all discontinuation events, reason for discontinuation in 10 patients (25%)].
Figure 4. Percentage of patients according to iPTH values defined by KDOQI guidelines at 3-month intervals during the study course.
There were 480 measurements for serum calcium and phosphorus during the 12-month study period. We detected 34 episodes in 17 patients (42.5% of patients, mean number of episodes for each patient: 2) in which serum calcium was between 10.2 and 11 mg/dL, whereas there were 15 episodes in 11 patients (27.5% of patients, mean number of episodes for each patient: 1.36) in which serum calcium was over 11 mg/dL. There were 50 episodes in 23 patients (57% of patients, mean number of episodes for each patient: 2.1) in which serum phosphorus was between 5.5 and 6 mg/dL, whereas there were 74 episodes in 26 patients (65% of patients, mean number of episodes for each patient: 2.8) in which serum phosphorus was over 6 mg/dL.
Serum iPTH levels did not show significant decrease in three patients. All were diagnosed with parathyroid adenoma. One patient accepted to undergo surgical parathyroidectomy. His serum parathyroid hormone levels reduced after surgical parathyroidectomy.
DISCUSSION
The main result of this was that paricalcitol was generally effective in reducing iPTH levels when compared with pretreatment values. Notably paricalcitol achieved this without a mean phosphatemic and/or calcemic effect despite relatively frequent discontinuation due to hyperphosphatemic and/or hypercalcemic surges in some patients.
This may have resulted in reduction and/or discontinuation of paricalcitol due to our lower thresholds of serum calcium and phosphorus. Moreover, monitoring iPTH levels once in every 3 months may have led to unnecessary interruption of the vitamin D treatment owing to overcorrection of serum iPTH levels below guideline targets and calcium and/or serum phosphorus derangements. One additional reason for frequent discontinuation due to hypercalcemia and/or hyperphosphatemia may be higher mean starting and maintenance doses of paricalcitol compared with previous studies.Citation15 None of the patients had to discontinue paricalcitol due to an adverse event other than calcium and/or phosphorus derangement.
Better achievement of KDOQI bone and mineral metabolism guidelines is related to lower mortality rates.Citation18 However, it is difficult to fulfill all guideline targets simultaneously.Citation19 This may be particularly true for patients with SHPT who are resistant to calcitriol treatment.
Although calcitriol is effective, therapy is frequently limited by hypercalcemia, hyperphosphatemia, and/or elevations in the Ca × P. In their double-blind, randomized, multicenter study, Sprague et al.Citation14 randomized hemodialysis patients with serum Ca × P <75 and a PTH level ≥300 pg/mL to receive either paricalcitol or calcitriol in a dose-escalating fashion for up to 32 weeks. Paricalcitol-treated patients achieved a ≥50% reduction from baseline PTH significantly faster than did the calcitriol-treated patients (p = 0.025) and achieved a mean reduction of PTH into a desired therapeutic range at approximately week 18, whereas the calcitriol-treated patients were unable to achieve this range. Moreover, paricalcitol-treated patients had significantly fewer sustained episodes of hypercalcemia and/or increased Ca × P than calcitriol patients. In a 12-week single-center, randomized, open-label study, Abdul Gafor et al.Citation11 showed that serum iPTH levels were significantly reduced only in the paricalcitol group but not in the calcitriol group, and serum calcium levels were significantly increased only in the calcitriol group. They found that serum phosphorus, alkaline phosphatase, and Ca × P were not different between the study groups. These results were reproduced in other studies.Citation11,14
It has been shown that paricalcitol treatment may be associated with lower mortality,Citation20 lower hospitalization rates, and in-hospital stayCitation21 compared with calcitriol treatment. These beneficial effects on hard outcomes may not be related only to effects on calcium–phosphorus metabolism but also depend on post-receptor differences between the molecules.Citation22
Previously Llach and YuddCitation15 studied the effects of paricalcitol in 37 calcitriol-resistant patients with SHPT in a long-term, prospective, open-label study. They found that the mean iPTH level (baseline, 901 ± 58 pg/mL) decreased rapidly during the initial 2 months and was 165 ± 24 pg/mL at 16 months. Mean calcium and phosphorus levels did not change significantly over the 16 months of paricalcitol therapy. They also reported that eight patients developed hypercalcemia, which was successfully managed by dietary counseling, phosphate-binder adjustment, and paricalcitol dose reduction. Six patients developed hyperphosphatemia; three patients responded adequately to dietary manipulation and phosphate binders, but three patients had repeated episodes. The main findings of this study are in line with our findings. However, we observed more hypercalcemia and hyperphosphatemia episodes, which also responded to dose reduction or discontinuation of paricalcitol treatment. Consequently, discontinuation rates of paricalcitol were higher in our study compared with the study of Llach et al.. We think that one of the main reasons for this difference may be higher thresholds for serum calcium and phosphorus values used by Llach and Yudd.Citation15 They withheld paricalcitol dose if serum calcium levels increased to >12.0 mg/dL or symptomatic hypercalcemia was suspected. Likewise paricalcitol was also withheld if the serum phosphorus levels increased to >7.5 mg/dL or Ca × P was >75. We think that our thresholds were more reasonable if we would like to prevent ectopic vascular calcification, which is considered one of the main determinants of cardiovascular mortality. Our thresholds are also more compliant with KDOQI guideline targets. Another explanation for our higher discontinuation rates may be the monitoring policy for serum iPTH levels once in every 3 months. This may have led to more fluctuations in serum iPTH, calcium, and phosphorus levels compared with monthly measurements in the study by Llach and Yudd.Citation15 However, there is social security reimbursement restriction regarding monthly measurement of iPTH in our country. We think that in contrast to calcitriol treatment, it would be more efficacious and reasonable to monitor serum iPTH levels on a monthly basis rather than at 3-month intervals. This policy may reduce undesired interruption of the vitamin D treatment and consequently may enhance beneficial treatment results in terms of mortality and calcium–phosphorus metabolism. Despite relatively frequent discontinuation of paricalcitol, almost all patients responded with an iPTH reduction compared with baseline pretreatment levels. Monthly measurement of iPTH may further enhance this beneficial effect without causing untoward phosphatemic and calcemic actions.
Our study has some limitations. First, we did not have a placebo group. However, we think that it was not feasible to include a placebo group because we were evaluating the effects of paricalcitol in calcitriol-resistant or calcitriol-intolerant patients. We chose to compare before and after treatment values with paricalcitol treatment. Second, we measured parathyroid hormone levels every 3 months. We may have missed some rapid changes in serum iPTH levels and this in turn may have led to over- or undertreatment with paricalcitol with regard to serum iPTH levels. However, current NKF/KDOQI guidelinesCitation17 recommend measurement of serum iPTH levels once every 3 months. We did not evaluate in all patients whether parathyroid adenoma is present at the outset of the study. However, we detected parathyroid adenoma in three unresponsive patients to paricalcitol. Actually Shuja and RajaCitation23 reported persistent severe hyperparathyroidism despite paricalcitol treatment in peritoneal dialysis patients. However, they did not provide the ratio of patients with parathyroid adenoma. In a recent study, Vulpio et al.Citation24 switched 30 hemodialysis patients with SHPT treated previously with calcitriol for at least 6 months to paricalcitol with a 1:4 conversion ratio. They divided the patients into two groups according to sonographically determined parathyroid gland sizes. After a 6-month treatment with paricalcitol, patients with lower parathyroid gland size achieved lower serum iPTH with lesser concomitant hypercalcemia and/or hyperphosphatemia and vice versa. Hence, the presence of parathyroid adenoma in our three patients who showed poor response to paricalcitol treatment also supports the findings of this latter study. It may be prudent to screen paricalcitol-unresponsive patients in terms of the presence of parathyroid adenoma. In these cases, adding cinacalcet to treatment regimen or surgical parathyroidectomy as the last resort may benefit patients.
In conclusion, this current study showed efficacy of paricalcitol in reducing serum iPTH levels without significant increases in mean serum calcium or phosphorus levels in patients with SHPT who are resistant to calcitriol treatment. In patients with SHPT who are resistant to calcitriol, an initial trial of paricalcitol may be beneficial and cost-effective before conducting costly investigations in search for a parathyroid adenoma. In patients who are also unresponsive to paricalcitol, investigation for parathyroid adenoma is warranted.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Salem MM. Hyperparathyroidism in the hemodialysis population: A survey of 612 patients. Am J Kidney Dis. 1997;29(6):862–865.
- Khan S. Vitamin D deficiency and secondary hyperparathyroidism among patients with chronic kidney disease. Am J Med Sci. 2007;333(4):201–207.
- Friedman EA. Consequences and management of hyperphosphatemia in patients with renal insufficiency. Kidney Int Suppl. 2005; 67(Suppl. 95):S1–S7.
- Moe SM, Drueke TB. Management of secondary hyperparathyroidism: The importance and the challenge of controlling parathyroid hormone levels without elevating calcium, phosphorus, and calcium-phosphorus product. Am J Nephrol. 2003;23(6):369–379.
- Cunningham J, Zehnder D. New vitamin D analogs and changing therapeutic paradigms. Kidney Int. 2011;79(7):702–707.
- Slatopolsky E, Cozzolino M, Finch JL. Differential effects of 19-nor-1,25-(OH)(2)D(2) and 1alpha-hydroxyvitamin D(2) on calcium and phosphorus in normal and uremic rats. Kidney Int. 2002;62(4):1277–1284.
- Andress DL. Intravenous versus oral vitamin D therapy in dialysis patients: What is the question? Am J Kidney Dis. 2001;38(5 Suppl. 5):S41–S44.
- Quarles LD, Yohay DA, Carroll BA, . Prospective trial of pulse oral versus intravenous calcitriol treatment of hyperparathyroidism in ESRD. Kidney Int. 1994;45(6):1710–1721.
- Brown AJ, Slatopolsky E. Vitamin D analogs: Therapeutic applications and mechanisms for selectivity. Mol Aspects Med. 2008;29(6):433–452.
- Cheng S, Coyne D. Paricalcitol capsules for the control of secondary hyperparathyroidism in chronic kidney disease. Expert Opin Pharmacother. 2006;7(5):617–621.
- Abdul Gafor AH, Saidin R, Loo CY, . Intravenous calcitriol versus paricalcitol in hemodialysis patients with severe secondary hyperparathyroidism. Nephrol (Carlton). 2009;14(5):488–492.
- Seeherunvong W, Nwobi O, Abitbol CL, Chandar J, Strauss J, Zilleruelo G. Paricalcitol versus calcitriol treatment for hyperparathyroidism in pediatric hemodialysis patients. Pediatr Nephrol. 2006;21(10):1434–1439.
- Drueke TB, McCarron DA. Paricalcitol as compared with calcitriol in patients undergoing hemodialysis. N Engl J Med. 2003;349(5):496–499.
- Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63(4):1483–1490.
- Llach F, Yudd M. Paricalcitol in dialysis patients with calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis. 2001;38(5 Suppl. 5):S45–S50.
- Capuano A, Serio V, Pota A, Memoli B, Andreucci VE. Beneficial effects of better control of secondary hyperparathyroidism with paricalcitol in chronic dialysis patients. J Nephrol. 2009;22(1):59–68.
- KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl. 3):S1–S201.
- Kimata N, Albert JM, Akiba T, . Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: The Japan dialysis outcomes and practice patterns study. Hemodial Int. 2007;11(3):340–348.
- Arenas MD, Alvarez-Ude F, Gil MT, . Application of NKF-K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease: Changes of clinical practices and their effects on outcomes and quality standards in three hemodialysis units. Nephrol Dial Trans. 2006;21(6):1663–1668.
- Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349(5):446–456.
- Dobrez DG, Mathes A, Amdahl M, Marx SE, Melnick JZ, Sprague SM. Paricalcitol-treated patients experience improved hospitalization outcomes compared with calcitriol-treated patients in real-world clinical settings. Nephrol Dial Trans. 2004;19(5):1174–1181.
- Rostand SG, Drueke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56(2):383–392.
- Shuja SB, Raja RM. Severe hyperparathyroidism despite paricalcitol therapy: One-year follow-up. Adv Perit Dial. 2003;19:231–235.
- Vulpio C, Maresca G, Distasio E, . Switch from calcitriol to paricalcitol in secondary hyperparathyroidism of hemodialysis patients: Responsiveness is related to parathyroid gland size. Hemodial Int. 2011. doi:10.1111/j.1542-4758.2010.00514.x. [Epub ahead of print].