Abstract
Background: Patients with proteinuria frequently show changes in thyroid hormone levels. Serum T3 depression predicts a negative outcome in chronic kidney disease (CKD) patients and may be associated with cardiovascular complications or chronic inflammation. Few studies have explored the relationship between thyroid hormone dysregulation and clinical outcome in patients with proteinuria. Methods: We reviewed thyroid function test results obtained from 211 patients with 24 h urinary protein excretion greater than 150 mg/day and found a correlation of thyroid hormone level with cardiovascular events and mortality. Results: T3 decreased with age (p = 0.001) and 24 h urine albumin (p = 0.028). Free T4 decreased in accordance with 24 h urine protein and serum creatinine (p = 0.034 and p = 0.033, respectively). In the Kaplan–Meier survival analysis, lower cumulative survival, higher cardiovascular events, and mortality were found in the low T3 group compared with the normal T3 group (p = 0.000, p = 0.013, and p = 0.001, respectively). In Cox regression analysis, we observed that, with low T3, decreased sodium, and old age, the incidence of cardiovascular complications (p = 0.000, p = 0.016, and p = 0.000, respectively), cardiovascular mortality (p = 0.000, p = 0.048, and p = 0.001, respectively), and all-cause mortality (p = 0.000, p = 0.017, and p = 0.000, respectively) increased. Conclusion: In CKD patients with proteinuria, low T3 concentration predicted all-cause mortality and cardiovascular event independently of the severity of proteinuria.
INTRODUCTION
In patients with proteinuria, urinary losses of thyroid hormones and thyroid hormone-binding proteins (THBPs) perturb circulating thyroid hormone levels.Citation1 Secondary synthesis of thyroid-stimulating hormone (TSH) increases markedly in nephrotic syndrome (NS); however, the excretion of albumin and THBP reduces the hormone-binding capacity and the actual concentrations of T3 and T4 in the blood.Citation1 In patients with renal dysfunction, the conversion of T4 to T3 is especially impaired, and thus, serum T3 decreases.Citation2
Subclinical dysthyroidism is defined by a combination of normal serum thyroid hormone with increased or decreased TSH. This condition presents a risk factor for the development and progression of cardiovascular complications.Citation3 In contrast, sick euthyroid syndrome (SES) is defined by hypothyroidism with the reduction of T3 and free T4 (fT4) without any increase in TSH. The clinical significance of SES remains unclear, since it occurs in patients with diverse conditions and diseases, and the thyroid does not appear to be dysfunctional.Citation4 In hemodialysis patients, free T3 (fT3) and IL-6 may indicate prognosis, and changes in T3 levels may be associated with cardiovascular complications or chronic inflammation.Citation5 Although patients with proteinuria frequently show changes in thyroid hormone levels, the relationships of these changes to cardiovascular complications and mortality are not known.
In this study, we discovered correlations between the changes in thyroid hormone caused by proteinuria with cardiovascular complications and mortality in patients with chronic kidney disease (CKD) and proteinuria.
SUBJECTS AND METHODS
We recruited subjects for this study from among the individuals who visited the nephrology department in our hospital from June 2006 to May 2009 with proteinuria as their chief complaint. We selected 211 patients (114 males and 97 females) who had proteinuria higher than 150 mg/day on 24 h urine test. This was a retrospective study that drew information about underlying diseases, hematology, serum biochemistry, serum thyroid hormones (T3, fT4, and TSH), and 24 h urine test from medical records. We evaluated the relationships among thyroid hormone levels, cardiovascular complications, and mortality rates.
Blood cell counts were calculated from absorbance using the ADVIA 2120 Hematology System (Siemens Healthcare, Berlin, Germany). Thyroid hormones were measured by direct chemiluminescence using the ADVIA Centaur (Bayer, Tarrytown, NY, USA). Serum protein, albumin, total cholesterol, triglycerides, blood urea nitrogen (BUN), and creatinine were measured by kinetic colorimetric assays using Roche-Hitachi Modular System (Roche Corporation, Basel, Switzerland).
Glomerular filtration rate (GFR) was calculated with the modification of diet in renal disease (MDRD) formula using serum creatinine. The low T3 group included patients without previous thyroid disease in whom serum T3 was less than 0.60 ng/mL and TSH was in normal range (0.35–5.50 μIU/mL). The low fT4 group included patients without previous thyroid disease in whom the fT4 was less than 0.83 ng/dL and TSH was in normal range (0.35–5.50 μIU/mL). Cardiovascular complications composed of three categories, including acute myocardial infarct, angina pectoris, and congestive heart failure, were assessed. Cerebrovascular diseases, such as stroke and cerebral infarction, were also assessed.
Patients were grouped in accordance with the presence or absence of nephrotic syndrome (NS), with or without thyroid dysfunction. Death and survival groups were compared using independent t-tests and the χ2-test. Cumulative survival rates and cardiovascular events were compared using the Kaplan–Meier method. Factors predicting cardiovascular complications and mortality were identified by Cox regression analysis. Calculations were performed using SPSS version 12 (SPSS Inc., Chicago, IL, USA), and p-values of less than 0.05 were considered to be statistically significant.
RESULTS
Clinical Characteristics of the Study Group
The mean age of the 211 patients was 57.46 ± 15.40 years and 114 patients (54.0%) were male. In the total study group, 121 patients (57.3%) had diabetes, 188 patients (89.1%) had hypertension, 75 patients (35.5%) had NS, 33 patients (15.6%) had reduced T3 levels, and 32 (15.2%) had reduced fT4 levels. The cardiovascular events that were found in 41 patients were classified as acute myocardial infarct in 16 patients (7.6%), angina pectoris in 13 patients (6.1%), and congestive heart failure in 12 patients (5.6%).
Serologic Tests and 24 h Urine Tests
The average leukocyte count was 8117.06 ± 3211.03/L; hemoglobin count was 10.13 ± 2.14 g/dL; and platelet count was 257.43 ± 95.96 × 103/L. In serum electrolyte tests, sodium was 139.45 ± 4.67 mmol/L; potassium was 4.66 ± 4.08 mmol/L; BUN was 47.16 ± 25.78 mg/dL; creatinine was 4.15 ± 3.28 mg/dL; and the GFR was 29.84 ± 29.69 mL/min. Serum total protein was 6.17 ± 0.99 g/dL; albumin was 3.31 ± 0.78 g/dL; total cholesterol was 198.75 ± 109.04 mg/dL; triglycerides were 167.87 ± 93.99 mg/dL; uric acid was 7.45 ± 2.40 mg/dL; and C-reactive protein (CRP) was 1.83 ± 3.50 mg/dL. In thyroid function tests, T3 was 0.93 ± 0.35 ng/mL; fT4 was 1.13 ± 0.34 ng/dL; and TSH was 1.85 ± 1.40 μIU/mL. In the 24 h urine test, the total protein concentration was 3183.62 ± 2926.97 mg/day and albumin was 2154.43 ± 2044.35 mg/day.
Underlying Diseases and Serologic Factors in Patients Who Survived and Patients Who Died
The patients who died were significantly older than those who survived (p = 0.000). The frequencies of diabetes, hypertension, NS, and other underlying diseases did not differ between the two groups. As compared with survivors, the patients who died showed higher leukocyte counts (p = 0.028), lower hemoglobin counts (p = 0.008), sodium (p = 0.003), total cholesterol (p = 0.007), triglycerides (p = 0.000), T3 (p = 0.011), and 24 h urinary albumin (p = 0.001) ().
Table 1. Comparison of basal characteristics and study results between survival and death groups.
Underlying Diseases and Serologic Factors in Patients with and without Cardiovascular Events
The patients with cardiovascular events were significantly older than those without cardiovascular events (p = 0.001). The frequencies of diabetes, hypertension, NS, and other underlying diseases did not differ between the two groups. As compared with patients without cardiovascular events, patients with cardiovascular events showed lower sodium (p = 0.011) and decreased GFR_MDRD (p = 0.011) ().
Table 2. Comparison of basal characteristics and study results between normal and cardiovascular event groups.
Underlying Diseases and Serologic Factors in the Normal and Low T3 Groups
The groups with normal and low T3 levels did not differ significantly in age and the frequencies of underlying diseases, such as diabetes, hypertension, and NS. In serologic tests, the low T3 group showed lower levels of total protein (p = 0.041), albumin (p = 0.006), and fT4 (p = 0.001), but leukocytes (p = 0.032) and 24 h urine albumin/creatinine (p = 0.0193) were elevated ().
Table 3. Comparison of basal characteristics and study results between normal and low T3 groups.
Linear Regression Analysis of Factors in Thyroid Hormone Dysregulation
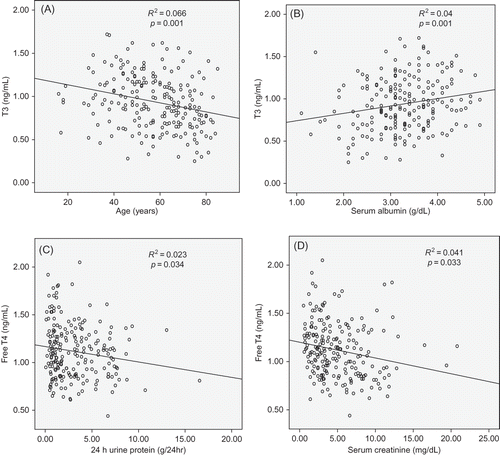
In linear regression analysis, T3 decreased with age (p = 0.001) and 24 h urine albumin (p = 0.028), and these effects were not influenced by the severity of serum creatinine. fT4 decreased in accordance with 24 h urine protein and serum creatinine (p = 0.034 and p = 0.033, respectively) and was not influenced by age. TSH did not change in accordance with proteinuria, age, and serum creatinine ().
Analysis of Causes of Death and Kaplan–Meier Survival Curves
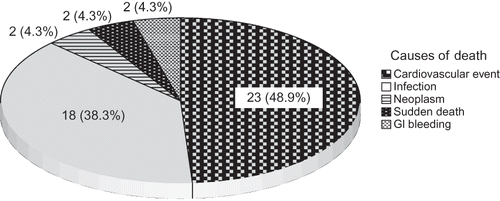
During the observation period, 47 of the 211 patients died. Twenty-three patients (48.9%) died from cardiovascular events, 18 patients (38.3%) from infection, 2 patients (0.8%) from cerebrovascular complications, 2 patients from neoplasm (4.3%), and 2 patients from sudden death (4.3%) ().
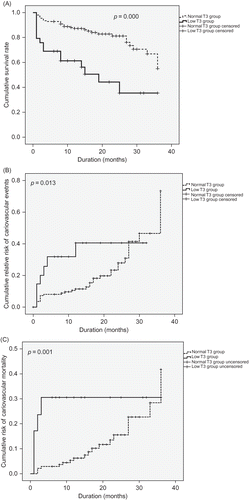
Based on the Kaplan–Meier method, lower cumulative survival, higher cardiovascular events, and mortality were found in the low T3 group compared with the normal T3 group (p = 0.000, p = 0.013, and p = 0.001, respectively) ().
Cox Regression Analysis of Factors in Cardiovascular Complications and Mortality
In the Cox regression analysis, sodium, total cholesterol, hematocrit, age, low T3, 24 h urine albumin, GFR_MDRD, sex, diabetes, and hypertension affect all-cause mortality, cardiovascular event, and mortality. We observed that with low T3, decreased sodium, and old age, the incidence of cardiovascular complications (p = 0.000, p = 0.016, and p = 0.000, respectively), cardiovascular mortality (p = 0.000, p = 0.048, and p = 0.001, respectively), and all-cause mortality (p = 0.000, p = 0.017, and p = 0.000, respectively) increased ().
Table 4. Cox regression analysis of factors affecting cardiovascular events and GFR_MDRD.
Table 5. Cox regression analysis of factors affecting cardiovascular mortality.
Table 6. Cox regression analysis of factors affecting all-cause mortality.
The Receiver Operating Characteristic Curve of Serum T3 Concentration and Mortality
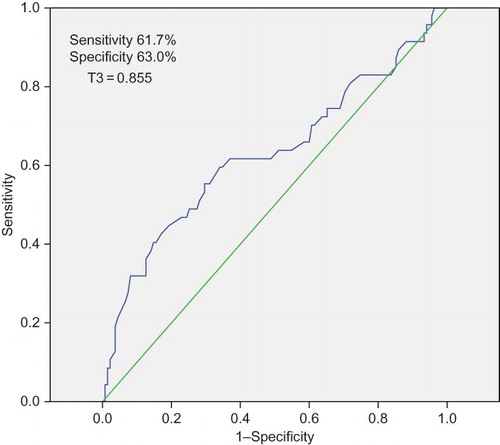
From the receiver operating characteristic (ROC) analysis of serum T3 and mortality, the area under the curve was found to be 0.571. Previously, the normal range of T3 was considered to be 0.60–1.81 ng/mL; however, in these patients with proteinuria, serum T3 lower than 0.855 ng/mL predicted mortality with 61.7% sensitivity and 63.0% specificity ().
DISCUSSION
Kidney disease and thyroid dysfunction show a close and consistent relationship. With persistent thyroid dysfunction, serum creatinine increases, while GFR, renal blood flow, salt reabsorption, and urine dilution ability decline.Citation2 In patients with renal failure, hyperthyroidism promotes glomerulosclerosis, proteinuria, and renal function decline through oxidative stress. In hypothyroidism, on the other hand, glomerular and tubular dysfunction tends to delay renal deterioration.Citation2
Kidney diseases may conversely compromise thyroid function. In CKD, dysregulation of the hypothalamus–pituitary–thyroid axis decreases T3 and T4 levels and increases TSH.Citation6 The progression of thyroid dysfunction depends on the GFR, with a critical value at approximately 64 mL/min/1.73 m2, according to the MDRD formula.Citation7 At this GFR or lower, uremia specifically impairs the conversion of T4 to T3. The reduction of T3 is reported to be associated with increased mortality in uremic patients, although the clinical significance of this is not clear.Citation8 The mechanism of T3 suppression in uremia may involve sustained malnutrition, chronic inflammatory reaction, and vascular injury.Citation9
In NS, urinary albumin excretion exceeds the capacity of albumin synthesis in the liver to maintain osmolality. This leads to thyroid hormone decline and THBP; however, a compensatory increase in TSH maintains serum thyroid hormone concentration, so that the actual incidence of hypothyroidism is as low as 1%.Citation1,2 One study reported significantly lower T3 and T4 levels and significantly higher TSH in pediatric patients with NS, as compared with a control group. These findings are consistent with the loss of thyroid hormone and its binding protein in urine.Citation10 In this study, T3 was positively correlated with serum albumin and negatively correlated with age; however, it did not change in accordance with serum creatinine. fT4 was primarily influenced by renal function; however, the severity of proteinuria did not change in accordance with age. TSH did not show the change in accordance with age, proteinuria, and renal function (). This finding may stem from complex phenomena associated with the impaired conversion of T4 to T3 that results from decreasing renal function.
Subclinical hypothyroidism, defined by normal thyroid hormone values with elevated TSH, may be a risk factor for mortality and a prognostic factor for cardiovascular disease.Citation11–13 In a study of 66,260 subjects drawn from the general population, the waist–hip ratios of hypothyroid patients were higher than those of controls. Thus, hypothyroidism may be related to obesity. Among patients with subclinical hypothyroidism, atherosclerosis risk factors, such as high total cholesterol and low-density lipoprotein, occurred more frequently than in the control group.Citation14 Abnormal lipid metabolism is closely related to increased levels of TSH, and the progression of subclinical hypothyroidism to actual hypothyroidism may accelerate atherosclerosis, which, in turn, increases the risk for cardiovascular complications.Citation15
Hypothyroidism, cardiovascular complications, and mortality may be similarly interrelated in renal failure. Zoccali et al.Citation5 reported a statistically significant negative correlation of serum T3 with mortality, determined from the survival curve of 200 patients who underwent hemodialysis. In that study, an increase in serum T3 concentration of 1 pg/mL corresponded to a 50% reduction in mortality in accordance with the hazard ratio. Thus, serum T3 may potently predict clinical outcome. In peritoneal dialysis patients, thyroid hormone levels correlated positively with serum albumin concentration and negatively with IL-6 and CRP, markers of chronic inflammatory response. The predictive power of serum T3 for mortality may be independent of traditional risk factors.Citation9,16 A study with 187 pre-dialysis patients reported an inverse correlation of T3 and fT3 with all chronic inflammation markers (IL-6, hs-CRP, and VCAM-1), and using the ROC curve analysis, it was found that T3 was a stronger prognostic factor for mortality than fT3.Citation17 In our study, the low T3 group experienced higher frequencies of cardiovascular events and mortality than normal T3 group (). Age may interact with serum T3 concentration and other factors in predicting the development of cardiovascular disease.
SES occurs with chronic diseases, such as chronic obstructive lung disease, diabetic ketoacidosis, ischemic heart disease, cancer, and renal failure.Citation4 In CKD, a systemic inflammatory condition, IL-6 participates in the IL-1-mediated suppression of thyroid function.Citation18 In a study on CKD patients, including dialysis patients, low T3 correlated with chronic inflammation and predicted mortality.Citation17,19 In our study, T3 predicted mortality and cardiovascular complications more reliably than fT4. Thus we suggest that T3 has greater clinical relevance than the subclinical hypothyroidism that results from impaired fT4 to T3 conversion in uremia.
Although the mechanism of subclinical hypothyroidism (SES) is not completely understood, it includes an elevation in type 3 deiodinase and a reduction in type 1, which result in T3 suppression, as well as an increase in type 2, which impairs the compensatory increase in TSH.Citation20,21 In patients with SES, administration of thyroid hormone (levothyroxine) does not resolve cardiovascular risk factors or complications.Citation22–24 Völzke et al.Citation25 discuss the difficulties in linking hypothyroidism or hyperthyroidism to mechanisms of mortality in terms of confounding parameters and selection biases. The results of our study did not further clarify the mechanism of SES, although SES predicted mortality from cardiovascular complications.
In our study, the patients who survived had higher total cholesterol and triglyceride levels than patients who died and higher amounts of total protein and albumin in 24 h urine (). This stands in contrast to the observation that protein loss in patients who die with metabolic syndrome or proteinuria is higher than those who survived in the general population. This relationship may stem from the chronic malnutrition and inflammation observed in CKD patients. So, kidney function should be considered in the analysis of such factors.
In this study, we explored the interrelationship between proteinuria and thyroid function. We found that among patients with kidney disease and proteinuria, serum T3 concentration predicts cardiovascular complications, as well as mortality, and that this association is independent of the amount of proteinuria. The strength of this correlation justifies further study to reveal the underlying mechanisms and their clinical relevance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Halma C. Thyroid function in patients with proteinuria. Neth J Med. 2009;67:153.
- Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2009;160:503–515.
- Rodondi N, Bauer DC, Cappola AR, . Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health Study. J Am Coll Cardiol. 2008;52:1152–1159.
- Di Napoli M, Reda G, Zannoni G, Russo S, Morace G, Vasselli C. The euthyroid sick syndrome. Its incidence and clinical significance in an internal medicine department. Minerva Med. 1994;85:161–165.
- Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P. Low triiodothyronine and survival in end-stage renal disease. Kidney Int. 2006;70:523–528.
- van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: A review. Gen Comp Endocrinol. 2009;160:205–215.
- Woodward A, McCann S, Al-Jubouri M. The relationship between estimated glomerular filtration rate and thyroid function: An observational study. Ann Clin Biochem. 2008;45:515–517.
- Song SH, Kwak IS, Lee DW, Kang YH, Seong EY, Park JS. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant. 2009;24:1534–1538.
- Enia G, Panuccio V, Cutrupi S, . Subclinical hypothyroidism is linked to micro-inflammation and predicts death in continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2007;22:538–544.
- Ito S, Kano K, Ando T, Ichimura T. Thyroid function in children with nephrotic syndrome. Pediatr Nephrol. 1994;8:412–415.
- Iacoviello M, Guida P, Guastamacchia E, . Prognostic role of sub-clinical hypothyroidism in chronic heart failure outpatients. Curr Pharm Des. 2008;14:2686–2692.
- Duntas LH, Wartofsky L. Cardiovascular risk and subclinical hypothyroidism: Focus on lipids and new emerging risk factors. What is the evidence? Thyroid. 2007;17:1075–1084.
- Duggal J, Singh S, Barsano CP, Arora R. Cardiovascular risk with subclinical hyperthyroidism and hypothyroidism: Pathophysiology and management. J Cardiometab Syndr. 2007;2:198–206.
- Jung CH, Sung KC, Shin HS, . Thyroid dysfunction and their relation to cardiovascular risk factors such as lipid profile, hsCRP, waist hip ratio in Korea. Korean J Med. 2002;63:273–282.
- Ochs N, Auer R, Bauer DC, . Meta-analysis: Subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med. 2008;148:832–845.
- Kang EW, Nam JY, Yoo TH, . Clinical implications of subclinical hypothyroidism in continuous ambulatory peritoneal dialysis patients. Am J Nephrol. 2008;28:908–913.
- Carrero JJ, Qureshi AR, Axelsson J, . Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. J Intern Med. 2007;262:690–701.
- Abozenah H, Shoeb S, Sabry A, Ismail H. Relation between thyroid hormone concentration and serum levels of interleukin-6 and interleukin-10 in patients with nonthyroidal illness including chronic kidney disease. Iran J Kidney Dis. 2008;2:16–23.
- Fernández-Reyes MJ, Sánchez R, Heras M, . Can FT3 levels facilitate the detection of inflammation or catabolism and malnutrition in dialysis patients? Nefrologia. 2009;29:304–310.
- Koenig RJ. Modeling the nonthyroidal illness syndrome. Curr Opin Endocrinol Diabetes Obes. 2008;15:466–469.
- Wu SY, Green WL, Huang WS, Hays MT, Chopra IJ. Alternate pathways of thyroid hormone metabolism. Thyroid. 2005;15:943–958.
- Kaptein EM, Beale E, Chan LS. Thyroid hormone therapy for obesity and nonthyroidal illnesses: A systematic review. J Clin Endocrinol Metab. 2009;94:3663–3675.
- Palmieri EA, Fazio S, Lombardi G, Biondi B. Subclinical hypothyroidism and cardiovascular risk: A reason to treat? Treat Endocrinol. 2004;3:233–244.
- Bello G, Paliani G, Annetta MG, Pontecorvi A, Antonelli M. Treating nonthyroidal illness syndrome in the critically ill patient: Still a matter of controversy. Curr Drug Targets. 2009;10:778–787.
- Völzke H, Schwahn C, Wallaschofski H, Dörr M. Review: The association of thyroid dysfunction with all-cause and circulatory mortality: Is there a causal relationship? J Clin Endocrinol Metab. 2007;92:2421–2429.