Abstract
Background and objective: Prolonged corticosteroid (CS) use induces osteoporosis; the pathogenesis of this condition is multifactorial and includes CS-induced hypercalciuria. We investigated the course of hypercalciuria and related markers of bone metabolism parameters during and after the CS treatment. Materials and Methods: We recruited 42 patients who were taking at least 10 mg/day of methylprednisolone or an equivalent dose of CSs for at least 30 days. The 24-h urinary calcium and sodium, a spot urinary calcium/creatinine ratio, and urinary deoxypyridinoline were measured prior to the treatment, at day 7, at days 30–60, and after the cessation of the treatment. Additionally, the serum levels of phosphorus, calcium, alkaline phosphatase (ALP), albumin, creatinine, osteocalcin, and parathyroid hormone (PTH) were analyzed. Results: The 24-h urinary calcium excretion was significantly increased at day 7 (182.2 ± 158.6 mg/day; p < 0.001) and at days 30–60 (196.9 ± 167.8 mg/day; p < 0.001) compared with baseline (98.7 ± 88.1 mg/day) and returned to basal level after the cessation of the CSs (118.9 ± 90.2 mg/day; p = 0.725). The urinary deoxypyridinoline level was significantly higher at days 30–60 compared with basal level. The serum osteocalcin level was decreased at days 30–60 when compared with day 7. No significant changes were detected in the PTH, phosphorus, creatinine, and ALP levels. Conclusions: CS treatment induces hypercalciuria just after starting the treatment until the end of it. CS-induced hypercalciuria promptly improved after cessation of the treatment. By days 30–60, the excretion of urinary deoxypyridinoline was accompanied by hypercalciuria. The serum osteocalcin level was decreased at days 30–60 when compared with day 7.
INTRODUCTION
Corticosteroids (CSs) have been used for the long-term treatment of several inflammatory disorders. One of the most important side effects of prolonged CS use is osteoporosis.Citation1 The pathogenesis of osteoporosis caused by CSs is multifactorial and the effect of CSs on the bone mineral metabolism is one of the important pathogenesis mechanisms.Citation2 CSs can decrease calcium reabsorption from renal tubules, which increases the renal excretion of calcium.Citation3 Hypercalciuria should be routinely checked in patients on CS treatment as recommended in the guidelines.Citation4 There are several studies on CS-induced hypercalciuria. However, there are few studies regarding the course of hypercalciuria and related markers of bone metabolism during the CS treatment.
We investigated the incidence and course of hypercalciuria during treatment and after the cessation of steroids. We also analyzed some biochemical parameters including bone metabolism markers such as deoxypyridinoline, parathyroid hormone (PTH), osteocalcin, phosphorus, and alkaline phosphatase (ALP).
MATERIALS AND METHODS
Ethics approval was obtained from the Ethics Committee of Yuzuncu Yil University before the study. We recruited 42 patients [22 female, 20 male; mean age 35 ± 15.8 years (16–65); mean body mass index 24.47 kg/m2 (18–39)], who were older than 16 years and on at least 10 mg/day of methylprednisolone or an equivalent dose of CSs for at least 30 days. We excluded the patients who were previously on CS treatments; were on antiepileptic medications; were on heparin that may affect bone metabolism; had diabetes mellitus and/or hypothyroidism; immobile, pregnant, or breastfeeding; and were on hormone or osteoporosis treatment.
The diagnosis of the patients and the decisions of starting and cessation of CS treatment were made by the related medical department. The associated physicians for each patient managed the calcium and vitamin D treatment for osteoporosis. The patients’ medical history revealed that only one patient regularly took calcium and vitamin D supplements. The restriction of salt intake was recommended for all patients.
The medical diagnoses of the patients included immune thrombocytopenic purpura (ITP) (n = 8), hemolytic anemia (n = 5), systemic lupus erythematosus (n = 4), nephrotic syndrome (n = 4), hypophyseal insufficiency (n = 2), drug eruptions (n = 2), lichen planus (n = 1), type 1 autoimmune polyglandular syndrome (n = 1), hypereosinophilic syndrome (n = 1), chronic lymphocytic leukemia (n = 2), polymyositis (n = 1), ulcerative colitis (n = 1), and acute lymphoblastic leukemia (ALL) (n = 1). The mean CS dose was 51.75 mg/day of methylprednisolone.
The patients with hypophyseal insufficiency (n = 2) and type 1 autoimmune polyglandular syndrome (n = 1) require life-long CS treatment. These three patients were taking 12.5 mg/day of prednisone, which is equivalent to 10 mg/day of methylprednisolone. During the study, the patient with polymyositis died from respiratory insufficiency, one patient with ITP died from alveolar hemorrhage, the patient with ALL died from hypotensive shock, and the patient with hemolytic anemia died from cardiogenic shock. In addition, the three patients did not routinely come for check-ups. Hence, a total of 10 patients were excluded due to lack of post-treatment evaluations.
A 24-h urinary calcium and sodium excretion, a spot urinary calcium/creatinine ratio, and urinary deoxypyridinoline were checked at baseline, at day 7 following the treatment, after 30–60 days of treatment, and at first visit after the cessation of the treatment. In addition, the serum phosphorus, calcium, ALP, albumin, creatinine, osteocalcin, and PTH levels were analyzed.
The 24-h urine collection was started from the control day at 8:00 a.m. until the following morning at 8:00 a.m. The spot urine was obtained in the morning after the completion of the 24-h urine collection. The urine samples were analyzed by the enzymatic colorimetric method via the “Integra 800” device (Roche Diagnostics, Mannheim, Germany). For osteocalcin analysis, the blood sample was collected in the ethylenediaminetetraacetic acid (EDTA)-containing tubes in the morning, and then rapidly transferred to the lab with ice packs.
For the deoxypyridinoline measurement, the midstream urine specimens were obtained in the morning and were analyzed by the chemiluminescence method via the “Immulite 2000” device (Diagnostic Products Corp., Los Angeles, CA, USA). The PTH level was analyzed by the chemiluminescence method via “Immulite 2000” device as well. The creatinine, ALP, calcium, and phosphorus levels were analyzed from the fasting blood samples by the colorimetric method via the “Modular” device.
The statistical analysis of the data was performed using SPSS software v.15.0 (SPSS Inc., Chicago, IL, USA). The related groups were analyzed using the paired samples t-test and the analysis of variance (ANOVA) test. The results were expressed as mean ± SD. A p-value of less than 0.05 was considered as statistically significant.
RESULTS
The parameters analyzed during the study are summarized in .
Table 1. Means, standard deviations, and p-values of all parameters.
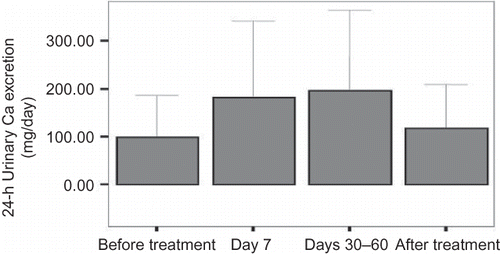
The 24-h urinary calcium excretion was significantly increased at day 7 and at days 30–60 compared with baseline (p < 0.001) (). There were no significant differences in the 24-h urinary calcium excretion between baseline and after cessation of the treatment and between day 7 and days 30–60.
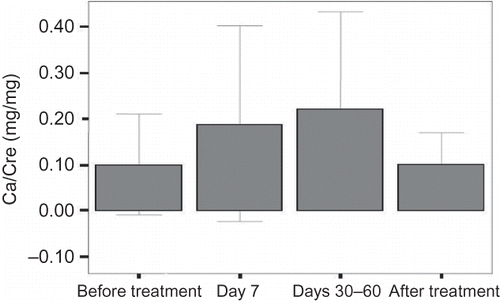
The calcium/creatinine ratio was also significantly increased at day 7 and at days 30–60 compared with baseline (p = 0.017 and p = 0.030, respectively) (). There were no significant differences in the calcium/creatinine excretion between baseline and after cessation of the treatment and between day 7 and days 30–60. The number of patients with hypercalciuria (more than 300 mg/day) was one (3.2%) at baseline, seven (23.3%) at day 7, six (30%) at days 30–60, and one (4.5%) after the cessation of the treatment. The incidence of hypercalciuria was significantly higher in patients on CS treatment compared with baseline and after the cessation of the treatment (p < 0.025).
The 24-h urinary sodium excretion was significantly increased at day 7 and days 30–60 compared with baseline (p < 0.05 for both). There were no significant differences between baseline and after cessation of the treatment and between day 7 and days 30–60.
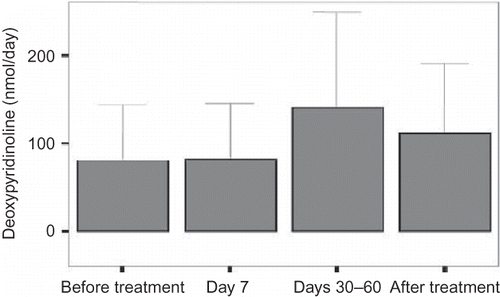
The urinary deoxypyridinoline excretion was significantly increased at days 30–60 (p < 0.05). There were no significant differences in the level of deoxypyridinoline between baseline and after cessation of the treatment and between day 7 and days 30–60 ().
There were no significant differences in the levels of albumin and calcium between baseline and days 30–60. During the course of the treatment, there were no significant differences in the levels of PTH, ALP, phosphorus, and creatinine.
DISCUSSION
In this study, we showed that CS treatment induces hypercalciuria just after starting the treatment until the end of it. In addition, CS-induced hypercalciuria promptly improved after cessation of the treatment. By days 30–60, the excretion of urinary deoxypyridinoline was accompanied by hypercalciuria.
There are several studies on CS-induced hypercalciuria. However, there are few studies regarding the course of hypercalciuria and related markers of bone metabolism during the CS treatment. The administration of 10 mg/day of prednisolone decreases the calcium absorption from intestineCitation5,6 and the administration of 20 mg/day or more of prednisolone increases the calcium excretion from kidney.Citation3,7 In Hodsman et al.Citation8 study, the comparisons of the low-dose prednisolone (10 mg/day) and the high-dose prednisolone (40 mg/day) effects on the bone mineral metabolism showed that the urinary calcium excretion was increased in the high-dose group. In our study, the minimum CS dose was 10 mg/day of methylprednisolone and there was no relationship between the CS dose and the calcium excretion. In Suzuki et al.Citation3 study involving 44 patients with collagen tissue disorders, the urinary calcium excretion was two times higher in the patients group compared with the controls; however, there were no significant differences in the levels of serum calcium, phosphorus, and ALP between the groups. Their results were compatible with our results.
We also evaluated the bone formation and bone resorption markers accompanied by hypercalciuria. Of these markers, urinary deoxypyridinoline increased after treatment revealing increased bone resorption, and osteocalcin decreased revealing decreased bone formation related to hypercalciuria. In the literature, most of the studies on the long-term CS side effects have been performed on patients with rheumatoid arthritis (RA). The bone resorption was high in patients with active RA, but CSs suppressed the disease process. In RA patients treated with CSs, bone resorption markers including hypercalciuria and urinary deoxypyridinoline excretion can be low, normal, or high.Citation9,10
The study on the comparison of glucocorticoids effects between the post-menopausal women with osteoporosis and without osteoporosis showed urinary calcium and urinary deoxypyridinoline excretions were higher in patients with osteoporosis. These findings may support the role of hypercalciuria and resorption in the pathogenesis of osteoporosis.Citation11
In a prospective study of the pulse methylprednisolone effects on bone metabolism involving 20 patients with RA receiving 1000 mg of methylprednisolone treatment, it was found that the excretion of urinary calcium which was higher during the treatment course was rapidly normalized after cessation of the treatment. In another study involving 30 patients with RA, the comparison of urinary calcium excretion between patients with intravenous methylprednisolone (1000 mg), patients with oral methylprednisolone (100 mg), and the control group showed an increase in the urinary calcium excretion in the intravenous methylprednisolone group within 6 h following the treatment, with no significant difference found in urinary deoxypyridinoline excretion between the groups.Citation12 Chiodini et al.Citation13 demonstrated that the urinary deoxypyridinoline excretion was significantly increased in patients with CS. In Hodsman et al.Citation8 study, the urinary deoxypyridinoline excretion was higher in patients with higher prednisolone therapy. In our study, we showed that CS treatment induces hypercalciuria just after starting the treatment until the end of it. In addition, the urinary deoxypyridinoline excretion was significantly higher at days 30–60 compared with baseline. There were no significant differences in the urinary deoxypyridinoline excretion between baseline and after cessation of the treatment and between day 7 and days 30–60.
The relationship between sodium and calcium excretion was shown in previous studies.Citation14,15 In our study, the 24-h sodium excretion increased at day 7 and at days 30–60. There were no significant differences in sodium and calcium excretion between baseline and after cessation of the treatment and between day 7 and days 30–60. In addition, there was no correlation between 24-h calcium excretion and 24-h sodium excretion.
In our study the serum albumin and calcium levels were significantly increased at days 30–60. This increase may be correlated with the recovery from the primary disease. Serum calcium level was calculated as the total calcium level that is affected by the albumin level.
There are some limitations in our study. They are (1) the lack of control group; (2) a decrease in the number of patients; (3) most of our patients were on the relatively short-term CS treatment, and thus we could not observe any long-term effects of CS treatment; and (4) our study population was heterogeneous.
In conclusion, patients treated with CSs can develop hypercalciuria starting from the beginning of the treatment to the end of the treatment. The urinary deoxypyridinoline excretion was accompanied by hypercalciuria by days 30–60 following the treatment. We believe that hypercalciuria should be routinely checked in patients on CS treatment. Further comprehensive studies should be performed to gain better insights into the long-term effects of CSs on calcium and bone metabolism.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20:629–650.
- Canalis E, Giustina A. Glucocorticoid-induced osteoporosis: Summary of a workshop. J Clin Endocrinol Metab. 2001;86:5681–5685.
- Suzuki Y, Ichikawa Y, Saito E, Homma M. Importance of increased urinary calcium excretion in the development of secondary hyperparathyroidism of patients under glucocorticoid therapy. Metabolism 1983;32:151–156.
- American College of Rheumatology Task Force on Osteoporosis Guidelines. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheum. 1996;39:1791–1801.
- Feldman D, Krishnan AV. Glucocorticoid effects on calcium metabolism and bone in the development of osteopenia. In: Christiansen C, Johansen JS, Riis BJ, eds. Osteoporosis. Copenhagen; 1987:1106.
- Rickers H, Deding A, Christiansen C, Rodbro P, Neastoft J. Corticosteroid-induced osteopenia and vitamin D metabolism. Effect of vitamin D2, calcium phosphate and sodium fluoride administration. Clin Endocrinol. 1982;16:409–415.
- Lund B, Storm TL, Lund B, . Bone mineral loss, bone histomorphometry and vitamin D metabolism in patients with rheumatoid arthritis on long-term glucocorticoid treatment. Clin Rheumatol. 1985;4:143–149.
- Hodsman AB, Toogood JH, Jennings B, Fraher LJ, Baskerville JC. Differential effects of inhaled budesonide and oral prednisolone on serum osteocalcin. J Clin Endocrinol Metab. 1991;72:530–540.
- Lems WF, Gerrits MI, Jacobs JW, van Vugt RM, van Rijn HJ, Bijlsma JW. Changes in (markers of) bone metabolism during high dose corticosteroid pulse treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 1996;55:288–293.
- Hall GM, Spector TD, Delmas PD. Markers of bone metabolism in postmenopausal women with rheumatoid arthritis. Effects of corticosteroids and hormone replacement therapy. Arthritis Rheum. 1995;38:902–906.
- Adams JS, Wahl TO, Lukert BP. Effects of hydrochlorothiazide and dietary sodium restriction on calcium metabolism in corticosteroid treated patients. Metabolism 1981;30:217–221.
- Need AG, Philcox JC, Hartley TF, Nordin BE. Calcium metabolism and osteoporosis in corticosteroid-treated postmenopausal women. Aust N Z J Med. 1986;16:341–346.
- Chiodini V, Carnevale M, Torlontano S, . Alterations of bone turnover and bone mass at different skeletal sites due to pure glucocorticoid excess: Study in eumenorrheic patients with Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83:1863–1867.
- King JS, Jackson R, Ashe B. Relation of sodium intake to urinary calcium excretion. Invest Urol. 1964;1:555–560.
- Modlin M. The interrelation of urinary calcium and sodium in normal adults. Invest Urol. 1966;4:180–189.