Abstract
Patients with chronic renal disease have a high prevalence of oxidative stress (OS), which is associated with the cardiovascular complications occurring in this population. The restoration of kidney function after kidney transplantation (KT) can lead to reduction in the metabolic abnormalities and elimination of the OS. Time-dependent changes in OS-related markers and specific kidney function and metabolic parameters were evaluated in patients (N = 39; 23 males; 16 females; mean age = 57 ± 10 years) before (day 0) and after KT (day 1, 7, 30, 90, and 180) to monitor the graft. In particular, total antioxidant capacity (TAC), levels of advanced oxidation protein products (AOPP), lipid peroxidation as thiobarbituric acid-reactive substances (TBARS) and reduced glutathione (GSH); activities of glutathione peroxidase, catalase, and superoxide dismutase; and kidney function markers were measured. AOPP, TAC, and TBARS were significantly decreased, whereas GSH was significantly increased after KT. Antioxidant enzyme activities were not significantly changed during the monitored period after KT. Apropos specific kidney function markers and glomerular filtration significantly increased and creatinine level significantly decreased after transplantation. Changes in high-density lipoprotein cholesterol were also found. Our results show that successful KT results in normalization of the antioxidant status and lipid metabolism that is connected with both improved renal function and reduced cardiovascular complications.
INTRODUCTION
Patients with chronic kidney disease (CKD) have a higher risk of cardiovascular disease (CVD) than the general population.Citation1 It is well known that CKD patients are exposed to primary risk factors, such as hypertension (activation of renin–angiotensin system, sympathetic nervous system, increased circulatory blood volume, deterioration of vascular endothelial function), hyperuricemia, inflammation, and secondary risk factors, such as oxidative stress (OS), and these are critical conditions for CVD development.Citation2–4 Inflammation and OS are directly associated with the atherosclerotic process and they may influence not only the cardiovascular system but also the graft function.Citation5
OS plays a central role in the pathogenesis of cardiovascular complications in chronic renal failure patients. OS is a state of imbalance between excessive formation of oxidant compounds and the antioxidant defence system that initiates oxidative modification of biomolecules leading to cellular and tissue damage.Citation6 Kidney transplantation (KT) is one treatment option for chronic renal failure patients when dialysis fails. Alone, KT is accompanied by induction of OS during the ischemia/reperfusion period in the course of transplantation, and this can lead to primary non-function of transplanted graft during the posttransplantation period or development and progression of chronic allograft nephropathy/shortening of the graft life.Citation7 The successful accomplishment of the early posttransplantation period contributes to the normalization of many metabolic disorders. The renormalization of OS after KT is not completely understood. Still discussed is whether the immunosuppressives that are the components of therapeutic protocols could contribute to OS.Citation8,9 Alterations to the oxidative status during and after KT have been intensively studied. Detailed examination of the oxidative status changes mainly after KT could lead to the development of therapeutic strategies for improving the function and prolonging the lifetime of the graft.
The aim of our trial was to study the alterations in OS-related markers, mainly antioxidant enzymes and specific kidney function markers before KT and during the posttransplantation period.
MATERIALS AND METHODS
Study Design
A prospective, randomized, 6-month, single-center study was designed to assess the time course of changes in OS-related parameters and specific kidney function markers before KT and in posttransplantation period. The study was conducted according to ICH GCP guidelines at the University Hospital Olomouc. The study protocol was approved by the Ethics Committee of the University Hospital and the Faculty of Medicine and Dentistry. All participants signed the informed consent before any study procedure was initiated. The study took place from November 2008 to December 2010 at the Department of Internal Medicine III, University Hospital.
Patients
Thirty-nine patients (23 males; 16 females; mean age = 57 ± 10 years) who underwent KT were included in the study. The causes of end-stage renal disease were as follows: chronic glomerulonephritis (n = 13), chronic tubule-interstitial nephritis (n = 5), autosomal dominant polycystic kidney disease (n = 4), diabetic nephropathy (n = 7), nephrosclerosis (n = 5), and unknown cause (n = 5) (). Patients undergoing systemic immunosuppressive therapy for reasons other than KT, patients with malignant disease or significant, uncontrolled concomitant infections, and female patients who were pregnant or breast-feeding were excluded. None of the patients were taking vitamin supplements (folic acid, vitamin C or E), and all were above 18 years of age. No participants had active viral hepatitis B or C. Patients were treated with calcineurin inhibitors cyclosporine A or tacrolimus combined with mycophenolate mofetil and corticosteroids. The initial daily dosage of cyclosporine A was 3 mg/kg, divided into two doses. The target cyclosporine A blood levels were 200–300 ng/mL at month 1 and 100–200 ng/mL at month 6. Cyclosporine A level was measured using fluorescence polarization immunoassay Architect Cyclosporine Reagent kit (Abbott Diagnostics, Prague, Czech Republic). The initial daily dosage of tacrolimus was 0.1 mg/kg, divided into two doses. The target blood tacrolimus levels were 5–15 ng/mL at months 1 and 4–10 ng/mL at month 6. Tacrolimus levels were measured using microparticle enzyme immunoassay ARC Tacrolimus Reagent Kit (Abbott Diagnostics). The daily dose of mycophenolate mofetil was 20 mg/kg. Prednisone was progressively tapered to reach a daily dose of 20 mg at day 1, 15 mg at month 3, and 5 mg at month 6. The dose given to each patient to reach the required serum levels can be considered for standardized as well as the duration of the therapy, which was equal for each subject. No induction immunosuppression with anti-thymocyte globulin was administered.
Table 1. Characteristics of the study population.
Sample Collection and Preparation
The blood samples were obtained one day before KT and on days 1, 7, 30, 90, and 180 after transplantation. Samples were collected from the patients after overnight fasting from antecubital vein into Vacuette® serum and K3 EDTA tubes (Greiner Bio-One, Kremsmünster, Austria). Basic biochemical and hematological parameters were determined in all samples. Serum, plasma, and isolated erythrocytes were used for the determination of OS parameters.
Clinical Chemistry and Hematology
Biochemical parameters [urea, creatinine, total protein, albumin, C-reactive protein, uric acid, and high-density lipoprotein (HDL)] were determined by commercial kits (Roche, Mannheim, Germany) using a Modular Analytics Evo analyser (Roche). Glomerular filtration was estimated using Modification of Diet in Renal Disease formula.
Low-density lipoprotein (LDL) level was estimated indirectly from measurements of total cholesterol, triacylglycerol, and HDL by means of the Friedewald’s equation.
Hematological parameters, erythrocyte number, hemoglobin, and hematocrit were analyzed using Coulter LH 750 (Beckman Coulter, Fullerton, CA, USA) and Sysmex XE-5000 (Toa Sysmex, Kobe, Japan) in the laboratories of the University Hospital Olomouc.
Oxidative Stress Parameters
Advanced oxidation protein products (AOPP) were measured using the spectrophotometric method according to Witko-Sarsat.Citation10 Total antioxidant capacity (TAC) was determined using a TAC assay kit (Randox Laboratories Ltd., Crumlin, UK). The amount of thiobarbituric acid-reactive substances (TBARS) was determined using the thiobarbituric acid reaction method.Citation11 The level of glutathione (GSH) was determined according to Sedlak and LindsayCitation12 using Ellman’s reagent. Superoxide dismutase (SOD) activity was measured using the indirect spectrophotometric method based on the generation of by a mixture of nitro blue tetrazolium, NADH, and phenazine methosulfate.Citation13 Glutathione peroxidase (GPX) activity was assayed spectrophotometrically at 340 nm by the modified method of Tappel.Citation14 Catalase (CAT) activity was assessed according to Beers and Sizer.Citation15
Statistical Analysis
All values were expressed as medians, first and third quartile. The data were statistically analyzed using the Wilcoxon paired test with Bonferroni correction of significance levels, Spearman correlation coefficient, and Mann–Whitney U-test. Data were applied to determine the statistically significant difference between values of parameters on the day before (day 0) and after KT (days 1, 7, 30, 90, and 180). Graphs of empirical cumulative distribution functions are used as graphic illustration of differences in progression during the 6 months after KT. The SPSS v.15 statistical package (SPSS Inc., Chicago, IL, USA) was used for all analyses. The level of significance was set at 5%.
RESULTS
Hematological and Biochemical Parameters
A significant decrease in plasma creatinine level and increase in glomerular filtration were observed 30 days after KT (, ). In the two shortest monitored periods (days 1 and 7) creatinine level rapidly decreased to 65% and 41%, respectively, of the value before KT. The changes in glomerular filtration were not as dramatic as in the case of creatinine. On day 1, the glomerular filtration stayed nearly unchanged, but by day 7 glomerular filtration increase was observed in more than 50% of patients. The improvement in glomerular filtration continued in the following intervals, and 3 and 6 months after KT the maximal values of glomerular filtration (1.02 and 0.98 mL/s) were achieved. Urea level decreased on day 1 after KT, but its concentration on day 7 after KT was surprisingly higher than before transplantation. Thirty days after KT, the urea level was reduced to the normal value (8.7 mmol/L). A similar trend was found in the case of uric acid ().
Figure 1. The plasma creatinine level (A) and glomerular filtration (B) during posttransplantation period. The data are expressed as differences of the value after KT (days 1, 7, 30, 90, and 180) and the value before KT (day 0).
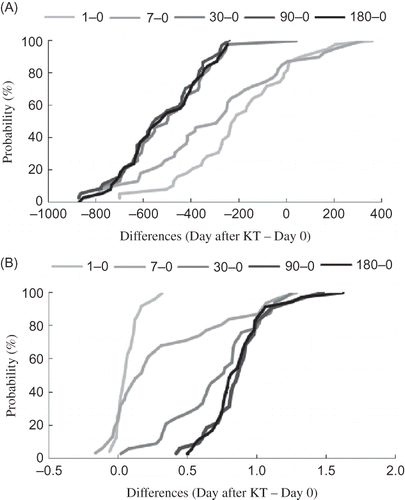
Table 2. Hematological and biochemical biomarkers before and after renal transplantation.
Changes in other biochemical and hematological parameters are shown in . Immediately after KT (days 1 and 7) the erythrocyte count, hemoglobin, and hematocrit were significantly reduced; however, at 1 month after KT all the parameters slightly increased. Normalization was found at the following time point (90 days after KT). Decline of total protein and albumin was observed after KT. However, 30 days after surgery their levels reached the values before KT. Further, KT showed a beneficial effect on lipid metabolism. Although 7 days after transplantation HDL-cholesterol was reduced compared with its level before KT (0.88 vs. 1.19 mmol/L), in the following intervals its concentration significantly increased. For LDL-cholesterol, statistically significant (p < 0.05) modification was found only on day 7 after KT (). At this time point, it was reduced to 88% of its value before KT. However, in the subsequent monitored periods (days 30, 90, and 180) LDL-cholesterol level increased nonsignificantly. The highest level of LDL-cholesterol (3.30 mmol/L) was found 30 days after KT, then moderately decreased but stayed higher than before KT.
Parameters of Inflammation and Oxidative Stress
Immediately after KT (days 1 and 7) C-reactive protein level was significantly increased (6.3- and 3.3-fold, respectively, of the value on day 0), but its concentration declined in the following monitored periods and was lower than that before KT ().
The AOPP and TAC in plasma and TBARS, GSH, CAT, GPX, and SOD in erythrocytes were used for the assessment of OS after KT. AOPP levels were significantly decreased in all monitored intervals compared with the level before KT (). After direct strong reduction (66.4 mol/L at day 1 vs. 173.1 mol/L at day 0), AOPP levels moderately increased in the following monitored intervals. However, the values did not reach pretransplantation levels (A). Reduction in TAC was observed in the whole 6-month monitored period of transplanted patients. As shown in , immediately after renal transplantation (day 1) TAC was markedly decreased and stayed reduced in all subsequent periods.
Figure 2. The long-term alteration of selected parameters of oxidative stress: advanced oxidation protein products (A), total antioxidant capacity (B), erythrocyte lipid peroxidation (C), and erythrocyte reduced glutathione (D) during posttransplantation period. The data are expressed as differences of the value after KT (days 1, 7, 30, 90, and 180) and the value before KT (day 0).
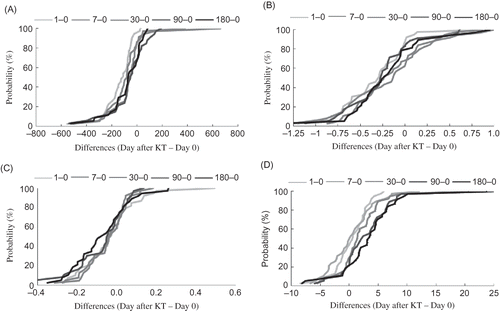
Table 3. Oxidative stress biomarkers before and after renal transplantation.
The expected accumulation of TBARS and depletion of GSH relating to ischemia/reperfusion during KT in the early monitored intervals (days 1 and 7) were not observed. Conversely, a slight reduction in TBARS level (C) and increase in GSH levels (D) in erythrocytes were found during the whole studied posttransplantation period. The highest content of GSH in erythrocytes (11.59 mol/L) was detected at month 6 after KT. Similarly, the maximal reduction of lipid peroxidation (TBARS) was found on day 180 after the surgery.
Activities of antioxidant enzymes CAT, SOD, and GPX that play an important role in the detoxification of reactive oxygen species in the body were surprisingly not significantly changed after KT. Only in CAT activity, nonsignificant decrease was observed during the first week after KT ().
DISCUSSION
Several studies have investigated the effects of OS and antioxidant status on graft recipients after KT.4,8,10,16–24 Our earlier trials were focused on changes in OS caused by calcineurin inhibitors in the posttransplantation period by evaluation of AOPP levels.Citation24,16 In this study, we examined the effects of KT on selected components of the antioxidant defence system in parallel with biochemical and hematological parameters over 6 months after transplantation. Our results show a substantial improvement in biochemical and hematological biomarkers, particularly glomerular filtration, creatinine, uric acid, urea, and C-reactive protein levels () in all graft recipients after renal transplantation. These results reflect the regeneration of renal function and are in agreement with previous reports.Citation5,17
The main goal of this study was the examination of changes in enzymatic (SOD, GPX, CAT) and nonenzymatic (GSH) antioxidants, TAC, and the interaction products of oxidants (reactive oxygen species) and biomolecules, AOPP and TBARS, as published information is controversial due to the study design. Earlier studies on the antioxidant defence system after KT mostly involved a shorter time period than our trial. Zahmatkesh et al.Citation8 focused on OS and antioxidant status (GSH, SOD, TBARS, TAC, and uric acid) over 2 weeks after KT. They found a decrease in TBARS and TAC, increase in SOD activity (28%), and nonsignificant changes in erythrocyte GSH level. Joo et al.Citation25 recently reported that 5 days after successful KT, serum lipid peroxidation products were significantly elevated, but 1 year after KT the lipid peroxidation was lower than before transplantation. Apropos the reduction in TBARS and TAC, we found similar results. However, we observed increase in erythrocyte GSH levels () but we found no significant variations in SOD activity. In other trials, changes in GSH metabolism, including enzymes important for GSH synthesis and regeneration especially GPX, glutathione reductase, and glutathione transferase over 2 weeks after KT were studied.Citation18 Two days after KT, a decrease (25%) in erythrocyte GSH content was observed. However, 2 weeks after transplantation, GSH levels increased to 1.8-fold the level before KT. Similar to our findings, De Vega et al.Citation18 observed no changes in GPX activity. Pe´rez Fernandez et al.Citation19 reported a decrease in SOD activity immediately (2 days) after KT and then its increase to nearly the value before transplantation at 2 weeks after KT. On the other hand, CAT activity remained nearly changed. A 28-day study described improvement in oxidative status parameters, TBARS decreased and SOD and GPX activities increased during the posttransplantation period.Citation20 As shown in , we found no effect of renal transplantation on the activity of either enzyme (CAT or SOD). There is also a disagreement on the results for lipid peroxidation. Vural et al.Citation20 observed a significant increase in lipid peroxidation products at 7 and 14 days after KT, but we found a significant decrease in the TBARS level 1 week after KT. Besides lipid peroxidation products, AOPP is a sensitive biomarker of macromolecule damage mediated by reactive oxygen species. In contrast to TBARS, it reflects the long-term situation in the body. AOPP levels were decreased in all monitored posttransplantation intervals, which is consistent with previously published results.Citation21 The obvious AOPP reduction directly after KT (day 1) can be explained by blood loss during surgery. However, its decrease in subsequent time points together with increased GSH and reduced TBARS confirmed that successful KT lowered OS and the antioxidant defence system is able to better eliminate reactive species generated in the body. Diminution of AOPP is consistent with the decline in protein carbonyl content and F2-isoprostanes, other long-term markers of OS that were reported by Simmons et al.Citation17 A TAC reduction after KT is also linked to better graft excretion of low molecular weight substances in urine that are not accumulated in body and cannot affect the TAC value. The modulation of TAC after KT is not connected with OS.Citation21
In summary, successful KT leads to improved graft function and decrease in OS. Even if kidney transplant recipients have a stable graft, they suffer from OS that is lower than in hemodialysis patients but higher than in the general population.Citation22,23 The evaluation of GSH or oxidatively modified biomolecules, such as lipid peroxidation products (TBARS) or oxidation protein products (AOPP), appears to be more sensitive than the evaluation of antioxidant enzymes activity or TAC. These parameters can be recommended for monitoring OS after KT and useful in the early therapeutic intervention. The improvement in the antioxidant status of kidney recipients can potentially prolong graft function and reduce the cardiovascular complications.
ACKNOWLEDGMENT
This work was supported by grants MSM 6198959216 and MZ CR (NS/9964-4).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Foley RN. Clinical epidemiology of cardiovascular disease in chronic kidney disease. J Ren Care. 2010;36(Suppl. 1):4–8.
- Himmelfarb J. Urenic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial. 2009;22(6):636–643.
- Kusano E. Mechanism by which chronic kidney disease causes cardiovascular disease and the measures to manage this phenomenon. Clin Exp Nephrol. 2011; 15(5):627–633.
- Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18(7):1272–1280.
- Cueto-Manzano AM, Morales-Buenrostro LE, González-Espinoza L, . Markers of inflammation before and after renal transplantation. Transplantation. 2005;80(1):47–51.
- Benzie IF. Evolution of antioxidant defence mechanisms. Eur J Nutr. 2000;39(2):53–61.
- Ha H, Park J, Kim YS, Endou H. Oxidative stress and chronic allograft nephropathy. Yonsei Med J. 2004;45(6):1049–1052.
- Zahmatkesh M, Kadkhodaee M, Mahdavi-Mazdeh M, . Oxidative stress status in renal transplant recipients. Exp Clin Transplant. 2010;8(1):38–44.
- Land WG. Ageing and immunosuppression in kidney transplantation. Exp Clin Transplant. 2004;2(2):229–237.
- Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, . Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49(5):1304–1313.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25(1):192–205.
- Ewing JF, Janero DR. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal Biochem. 1995;232(2):243–248.
- Tappel AL. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978;52:506–513.
- Beers Jr., RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133–140.
- Zadrazil J, Strebl P, Krejcí K, . Effect of different calcineurin inhibitors on AOPP and TAS after kidney transplantation. Clin Biochem. 2010;43(6):559–565.
- Simmons EM, Langone A, Sezer MT, . Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation. 2005;79(8):914–919.
- De Vega L, Férnandez RP, Mateo MC, Bustamante JB, Herrero AM, Munguira EB. Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren Fail. 2002;24(4):421–432.
- Pérez Fernandez R, Martín Mateo MC, De Vega L, Bustamante J, Herrero M, Bustamante Munguira E. Antioxidant enzyme determination and a study of lipid peroxidation in renal transplantation. Ren Fail. 2002;24(3):353–359.
- Vural A, Yilmaz MI, Caglar K, . Assessment of oxidative stress in the early posttransplant period: Comparison of cyclosporine A and tacrolimus-based regimens. Am J Nephrol. 2005;25(3):250–255.
- Antolini F, Valente F, Ricciardi D, Baroni M, Fagugli RM. Principal component analysis of some oxidative stress parameters and their relationships in hemodialytic and transplanted patients. Clin Chim Acta. 2005;358(1–2):87–94.
- Antolini F, Valente F, Ricciardi D, Fagugli RM. Normalization of oxidative stress parameters after kidney transplant is secondary to full recovery of renal function. Clin Nephrol. 2004;62(2):131–137.
- Minz M, Heer M, Arora S, Sharma A, Khullar M. Oxidative status in stable renal transplantation. Transplant Proc. 2006;38(7):2020–2021.
- Štrebl P, Horčička V, Krejčí K, . Oxidative stress after kidney transplantation: The role of immunosuppression. Dial Transplant. 2010;39:391–394.
- Joo DJ, Huh KH, Cho Y, . Change in serum lipid peroxide as an oxidative stress marker and its effects on kidney function after successful kidney transplantation. Transplant Proc. 2010;42(3):729–732.
