Abstract
Background: Aliskiren, a novel direct renin inhibitor, is hypothesized to inhibit the renin–angiotensin– system. This study attempted to provide insight into this mechanism by examining the antihypertensive and renoprotective effects of aliskiren in hypertensive chronic kidney disease (CKD) patients. Methods: After recruitment, 43 hypertensive CKD patients (mean age, 53.7 years) began treatment of aliskiren. The patients were classified into high (over 30 mL/min/1.73 m2) estimated glomerular filtration rate (eGFR) group or low (under 30 mL/min/1.73 m2) eGFR group for comparison of measurements of various parameters over the 6-month observation period. Results: Systolic blood pressure/diastolic blood pressure of 150 mg/day group decreased to an average of 126.8 ± 21.6 mmHg/69.3 ± 15.1 mmHg (average decrease: −7.4/–8.3 mmHg) over the 6-month observation period, while that of 300 mg/day group significantly decreased to an average of 133.5 ± 14.0 mmHg/71.5 ± 11.7 mmHg (average decrease: −21.1/–14.6 mmHg). Urinary protein of all patients decreased slightly and insignificantly to 1.1 ± 1.7 g/gCr from 1.4 ± 2.5 g/gCr. The serum creatinine (Cr) level of all patients decreased from 1.81 ± 1.10 mg/dL to 1.78 ± 0.82 mg/dL. Although the serum Cr level of the high eGFR group decreased from 1.28 ± 0.45 mg/dL to 1.27 ± 0.57 mg/dL, that of the low eGFR group slightly but insignificantly increased from 2.49 ± 0.64 mg/dL to 2.69 ± 1.11 mg/dL. Conclusion: Administration of aliskiren exerts an antihypertensive effect on hypertensive CKD patients that may lead to a decrease in urinary protein and an improvement in renal functioning.
INTRODUCTION
Although it was once believed that the renin–angiotensin–aldosterone system (RAAS) was a simple circulation controlling element, it is now known that it is involved in various clinical conditions, including hypertension.Citation1 This conclusion has been supported by many large-scale clinical trials that found that suppression of the RAAS with aliskiren, a direct renin inhibitor, reduces cardiovascular events and provides organ protection.Citation2–6 Specifically, aliskiren has been found to exert a dose-dependent antihypertensive effect on hypertensive patients via a clear and lucid mechanism of action, namely inhibition of the downstream response by inhibition of the enzymatic activity of renin located upstream of the RAAS.Citation7–9
Previous research has found that the antihypertensive effect of aliskiren may be strengthened by combining its administration with that of other antihypertensive medications.Citation2,10–12 Interestingly, aliskiren has been found to provide protection to the heart and kidneys in a manner that does not depend on its antihypertensive effect and that is different from the mechanism by which conventional RAAS inhibitors act.Citation13–17 This study attempted to provide insight into this mechanism by further examining the antihypertensive effects of aliskiren and comparing its effects on urinary protein and renal functioning when administered as a single agent and when administered as part of a combined drug regimen to hypertensive patients with chronic kidney disease (CKD) due to various primary diseases.
SUBJECTS AND METHODS
This study examined 43 hypertensive CKD patients who visited the outpatient department of the study site for treatment. The data were collected via monthly measurement of blood pressure, biochemical examination of blood, and urinalysis over a 6-month observation period. The observation period began when the patients initiated aliskiren therapy at a dosage of 150 mg/day. If, after 1 month of this treatment regimen, their systolic blood pressure (SBP) was found to be higher than 140 mmHg or their diastolic blood pressure (DBP) higher than 90 mmHg during the contraction phase, their dosage was increased to 300 mg/day. This dosage was not changed during the 6-month observation period, without adding other antihypertensive medications to the treatment regimen. And no drugs prescribed for diabetes and/or dyslipidemia were changed during the 6-month observation period.
To analyze the extensive quantity of data collected over the observation period, one-way analysis of variance (ANOVA) was performed, followed by Tukey’s multiple comparison testing as a post hoc means of examining the ANOVA results. Statistical analyses were performed using GraphPad PRISM™ V5.0 (GraphPad Software, San Diego, CA, USA). All results that reached a p < 0.05 level of significance was considered statistically significant, and the value of each parameter was expressed in terms of mean ± standard deviation (mean ± SD). To examine the changes in urinary protein, a regression line was constructed by comparing the data collected for 6 months after treatment initiation to data collected for 6 months before treatment initiation. The estimated glomerular filtration rate (eGFR) for the Japanese population, which is calculated using the formula eGFR (mL/min/ 173 m2) = 194 × serum creatinine (Cr) − 1.094 × age − 0.287 × 0.736 (if female), was used to evaluate renal functioning at baseline, using the serum Cr level measured by an enzymatic method.Citation18 Based on the results, the patients were classified into a low (under 30 mL/min/1.73 m2) eGFR group or a high (over 30 mL/min/1.73 m2) eGFR group, and a regression line was constructed to compare the groups.
RESULTS
shows the background data collected from the 23 men and 20 women with an average age of 53.7 ± 15.6 years who participated in this study. The most common main primary disease was diabetes mellitus (DM; 13 patients or 30.2% of the sample), followed by IgA nephropathy (11 patients or 25.6%); nephrosclerosis (4 patients or 9.3%); focal segmental glomerular sclerosis (4 patients or 9.3%); autosomal dominant polycystic kidney (3 patients or 7%); toxemia of pregnancy (2 patients or 4.7%); minimal change nephrotic syndrome (2 patients or 4.7%); and rapidly progressive glomerulonephritis, membranous nephropathy, obesity nephropathy, or thin basement membrane disease (1 patient each or 2.3% of the sample each). At baseline, the treatment regimen of 11 patients who had been taking one hypertensive drug other than aliskiren was changed to a regimen of 150 mg/day of aliskiren only, while administration of 150 mg/day of aliskiren was added to the existing antihypertensive drug regimen of 32 patients. Based on the measurement of blood pressure after 1 month of treatment, the dosage of 16 patients was increased to 300 mg/day while 27 patients continued a regimen of 150 mg/day.
Table 1. Patients’ baseline characteristics (N = 43).
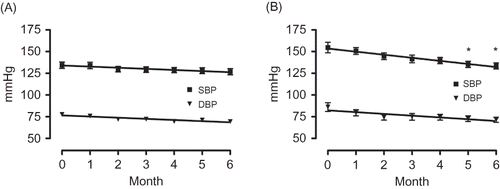
At initiation of aliskiren therapy, the average SBP/DBP at the contraction phase was 134.2 ± 24.1 mmHg/77.6 ± 16.5 mmHg in the 150 mg/day group and 154.6 ± 24.4 mmHg/86.1 ± 20.1 mmHg in the 300 mg/day group. All patients’ average serum Cr level at treatment initiation was 1.78 ± 0.82 mg/dL; eGFR was 49.7 ± 30.4 mL/min/1.73 m2; and urinary protein was 1.4 ± 2.5 g/gCr. A and B shows the changes in the consulting room blood pressure of the 150 mg/day and 300 mg/day groups, respectively, over the 6-month observation period. As can be observed, the average SBP/DBP of the 150 mg/day group insignificantly decreased to 126.8 ± 21.6 mmHg/69.3 ± 15.1 mmHg (an average decrease of −7.4/–8.3 mmHg) after 6 months of treatment while that of the 300 mg/day group significantly decreased to 133.5 ± 14.0 mmHg/71.5 ± 11.7 mmHg (an average decrease of −21.1/–14.6 mmHg).
Figure 1. Changes in the consulting room blood pressure of the 150 mg/day group (A) and the 300 mg/day group (B).
Note: *Significant according to the results of a one-way ANOVA (p = 0.0045) and Tukey’s multiple comparison testing (0 vs. 5, 0 vs. 6).
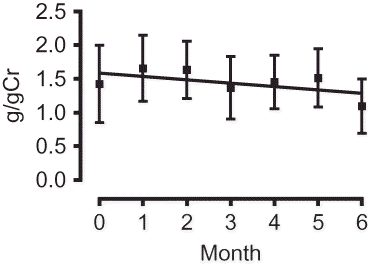
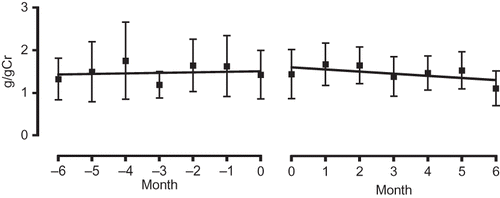
A and B shows the changes in the blood pressure of the high and low eGFR groups, respectively, over the 6-month observation period. As can be observed, the SBP/DBP of both groups showed a tendency to decrease, with that of the high eGFR group decreasing to an average of 122.4 ± 16.3 mmHg/66.7 ± 12.5 mmHg (an average decrease of −15.2/–12.9 mmHg) and that of the low eGFR group to an average of 126.1 ± 19.6 mmHg/67.2 ± 12.0 mmHg (an average decrease of −6.4 mmHg/–7.8 mmHg). shows the changes in urinary protein level after initiation of aliskiren therapy. As can be observed, the level decreased from 1.4 ± 2.5 g/gCr to 1.1 ± 1.7 g/gCr over the 6-month observation period, which, although not large, is a decrease nonetheless. When the gradient of the regression line constructed for the period 6 months before initiation of treatment to initiation of treatment was compared with that constructed for the period from initiation of treatment to 6 months after initiation of treatment, we found it to be −0.049, minus from 0.013 ().
Figure 2. Changes in the consulting room blood pressure of the high eGFR group (A) and the low eGFR group (B).
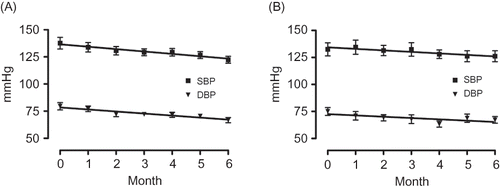
Figure 4. Comparison of gradient of the regression lines of urinary protein values before administration (−6 to 0 months; left panel) and after administration (0 to 6 months; right panel).
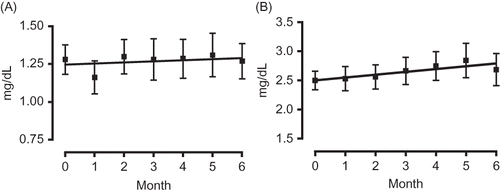
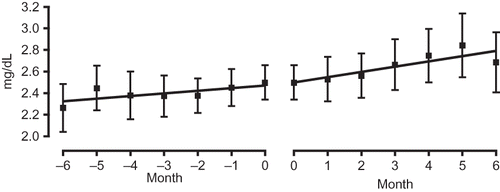
The results of biochemical examination of the blood indicate that aliskiren therapy did not have a significant effect on serum Cr levels, which decreased from an average of 1.81 ± 1.10 mg/dL to 1.78 ± 0.82 mg/dL over the 6-month observation period. Similar to the manner in which the effect of aliskiren therapy on blood pressure was evaluated, its effect on renal functioning was evaluated by comparing changes in eGFR over the 6-month observation period. In the high eGFR group, the serum Cr level slightly decreased from 1.28 ± 0.45 mg/dL to 1.27 ± 0.57 mg/dL (A). Although the results of ANOVA indicated insignificant differences, the serum Cr level of the low eGFR group increased from 2.49 ± 0.64 mg/dL to 2.69 ± 1.11 mg/dL, showing an upward trend (B). When the gradient of the regression line constructed for the period 6 months before initiation of treatment to initiation of treatment was compared with that constructed for the period from initiation of treatment to 6 months after initiation of treatment, the gradient was found to have decreased from 0.023 to 0.007 in the high eGFR group () but to have increased from 0.025 to 0.049 in the low eGFR group ().
Figure 6. Comparison of gradient of the regression lines of the serum Cr level of the high eGFR group before administration (−6 to 0 months; left panel) and after administration (0–6 months; right panel).
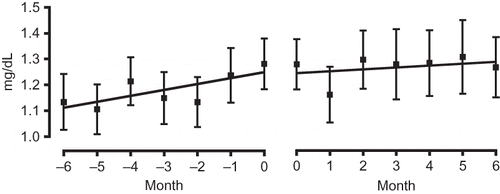
Figure 7. Comparison of gradient of the regression lines of the serum Cr level of the low eGFR group before administration (−6 to 0 months; left panel) and after administration (0 to 6 months; right panel).
No adverse events that could be problematical, such as changes in serum potassium concentrations, were observed among the patients.
DISCUSSION
The results of this study indicate that both sole administration of the direct renin inhibitor aliskiren and the addition of aliskiren administration to the current drug regimen of hypertensive CKD patients can exert a significant antihypertensive effect, regardless of eGFR value before aliskiren treatment initiation, if the dosage is sufficiently high. Specifically, the results indicate that the antihypertensive effect of aliskiren is only realized by administration of 300 mg/day, as administration of 150/mg day was found not to exert a significant hypertensive effect. This may be the reason many of the patients in the 150 mg/day group were experiencing relatively good control of blood pressure.
Previous research found that the addition of aliskiren administration to the administration of diuretics, calcium-channel blockers, angiotensin I-converting enzyme inhibitors, angiotensin receptor blockers, or beta blockers exerts a greater antihypertensive effect compared with simply increasing the dosage of the latter without adding aliskiren.Citation12,15,19 The findings of these studies support this research, suggesting that the addition of antihypertensive medications that act by mechanisms that differ from those of antihypertensive medications currently being administered results in further decreases in blood pressure. It is now necessary to determine when addition of aliskiren is warranted and to identify the combination of antihypertensive medications that result in the greatest decreases in blood pressure.Citation17,20,21
If eGFR decreases and the stage of CKD progresses, the degree of hypertension becomes more serious and antihypertensive medications hardly work. However, aliskiren has been found to have both a significant antihypertensive effect and a high degree of tolerability among Japanese hypertensive patients with renal dysfunction.Citation22 When the patients were divided into a mild (Stages 1–3) CKD group (eGFR: over 30 mL/min/1.73 m2) and a severe (Stage 4 and above) CKD group (eGFR: under 30 mL/min/1.73 m2) and their data compared, the effect of administration was not found to significantly differ. Although the mild CKD group experienced a slightly stronger antihypertensive effect, both groups experienced decreases in blood pressure and tolerated aliskiren to an equivalent extent. Although the background of the patients in this study differed from that of patients in previous studies, these findings support the prior research.Citation7,22
Regarding the effect of aliskiren on urinary protein, the study results indicate that aliskiren provides kidney protection by decreasing the urinary protein level without significantly changing the eGFR. Reduction in urine albumin-to-creatinine ratio (UACR) via the antihypertensive effect exerted by aliskiren has been reported in type II DM patients with hypertension, as well as reduction in UACR that does not depend on the antihypertensive effect in type II DM patients.Citation2,6 It is unknown whether the decrease in urinary protein experienced by the patients in this study depended on the antihypertensive effect of aliskiren or on another factor. Of the various hypotheses regarding the mechanism by which aliskiren decreases proteinuria without decreasing eGFR,Citation23 one proposes that aliskiren increases renal plasma flow, while another proposes that aliskiren decreases systemic blood pressure, thereby decreasing intraglomerular pressure and improving glomerular hyperfiltration.Citation16 It is anticipated that future research will clarify the mechanism by which aliskiren acts on renal functioning, as well as the characteristic effects of direct renin inhibitors.
Administration of aliskiren was found to decrease the serum Cr level of patients with an eGFR greater than 30 mL/min/1.73 m2 over the 6-month observation period, indicating that it can suppress elevated levels of serum Cr and thereby improve the renal functioning of these patients. We can think in a different way about this form of kidney protection of aliskiren shown by the decrease in proteinuria effect that we described above. A transient increase in serum Cr level has often been observed early in administration of RAAS inhibitors (angiotensin I-converting enzyme inhibitors and angiotensin receptor blockers).Citation24 It is commonly understood that RAAS inhibitors effectively expand the efferent glomerular arterioles but not the afferent glomerular arteriole, a mechanism that depends on decreasing the intraglomerular pressure and, by protecting the glomeruli, protects the kidneys.Citation25 However, several studies found that aliskiren is less likely to cause a rise of transient serum Cr level.Citation2,9 This finding accords with the results of this study and suggests that aliskiren provides kidney protection via a mechanism that differs from that of other RAAS inhibitors. However, the results of this study also indicate that administration of aliskiren to patients with an eGFR below 30 mL/min/1.73 m2 tends to increase serum Cr values. Thus, it is necessary to consider the possibility of adaptation when patients with severe renal dysfunction begin aliskiren therapy.Citation26
In conclusion, the results of this investigation into the effects of the direct renin inhibitor aliskiren indicate that its administration, whether as a single agent or in combination with other antihypertensive agents, exerts an antihypertensive effect in CKD patients with hypertension, leading to a decrease in proteinuria that likely improves renal functioning. As such, aliskiren administration may be an effective means of controlling hypertension in CKD patients.
Declaration of interest: The authors report on conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Berl T. Review: Renal protection by inhibition of the renin–angiotensin–aldosterone system. J Renin Angiotensin Aldosterone Syst. 2009;10(1):1–8.
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446.
- Schernthaner G. Dual inhibition with losartan and aliskiren: A promising therapeutic option for type 2 diabetic nephropathy? Nat Clin Pract Nephrol. 2008;4:656–657.
- Siamopoulos KC, Kalaitzidis RG. Inhibition of the renin–angiotensin system and chronic kidney disease. Int Urol Nephrol. 2008;40(4):1015–1025.
- Abassi Z, Winaver J, Feuerstein GZ. The biochemical pharmacology of renin inhibitors: Implications for translational medicine in hypertension, diabetic nephropathy and heart failure: Expectations and reality. Biochem Pharmacol. 2009;78(8):933–940.
- Parving H-H, Brenner BM, McMurray JJV, . Aliskiren trial in type 2 diabetes using cardio-renal endpoints (ALTITUDE): Rationale and study design. Nephrol Dial Transplant. 2009;24:1663–1671.
- Kushiro T, Itakura H, Abo Y, Gotou H, Terao S, Keefe DL. Aliskiren, a novel oral renin inhibitor, provides dose-dependent efficacy and placebo-like tolerability in Japanese patients with hypertension. Hypertens Res. 2006;29:997–1005.
- Peixoto AJ, Orias M. Is there a role for direct renin inhibitors in chronic kidney disease? Curr Opin Nephrol Hypertens. 2009;18(5):397–403.
- Schmieder RE, Philipp T, Guerediaga J, . Long-term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: A 12-month randomized, double-blind comparator trial with hydrochlorothiazide. Circulation. 2009;119:417–425.
- Pool JL, Schmieder RE, Azizi M, . Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens. 2007;20:11–20.
- Wiggins KJ, Kelly DJ. Aliskiren: A novel renoprotective agent or simply an alternative to ACE inhibitors? Kidney Int. 2009;76(1):23–31.
- Siragy HM. Rationale for combining a direct renin inhibitor with other renin–angiotensin system blockers. Focus on aliskiren and combinations. Cardiovasc Drugs Ther. 2011;25:87–97.
- Solomon SD, Appelbaum E, Manning WJ, . Effect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119:530–537.
- Morishita Y, Hanawa S, Chinda J, Iimura O, Tsunematsu S, Kusano E. Effects of aliskiren on blood pressure and the predictive biomarkers for cardiovascular disease in hemodialysis-dependent chronic kidney disease patients with hypertension. Hypertens Res. 2011;34(3):308–313.
- Moriyama T, Tsuruta Y, Kojima C, . Beneficial effect of aliskiren combined with olmesartan in reducing urinary protein excretion in patients with chronic kidney disease. Int Urol Nephrol. 2011.
- Siddiqi L, Oey PL, Blankestijn PJ. Aliskiren reduces sympathetic nerve activity and blood pressure in chronic kidney disease patients. Nephrol Dial Transplant. 2011 ;26:2930–2934.
- Trimarchi H. Role of aliskiren in blood pressure control and renoprotection. Int J Nephrol Renovasc Dis. 2011;4:41–48.
- Matsuo S, Imai E, Horio M, . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992.
- Alfie J, Aparicio LS, Waisman GD. Current strategies to achieve further cardiac and renal protection through enhanced renin–angiotensin–aldosterone system inhibition. Rev Recent Clin Trials. 2011;6:134–146.
- Chaudhary K, Nistala R, Whaley-Connell A. Is there a future for direct renin inhibitors? Expert Opin Investig Drugs. 2010;19(5):653–661.
- Siragy HM. A current evaluation of the safety of angiotensin receptor blockers and direct renin inhibitors. Vasc Health Risk Manag. 2011;7:297–313.
- Ito S, Nakura N, Le Breton S, Keefe D. Efficacy and safety of aliskiren in Japanese hypertensive patients with renal dysfunction. Hypertens Res. 2009;33:62–66.
- Tang SCW, Lin M, Tam S, . Aliskiren combined with losartan in immunoglobulin A nephropathy: An open-labeled pilot study. Nephrol Dial Transplant. 2011;1–6.
- Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin–angiotensin–aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;68(99):S57–S65.
- Weir MR. Effects of renin–angiotensin system inhibition on end-organ protection: Can we do better? Clin Ther. 2007;29(9):1803–1824.
- Venzin RM, Cohen CD, Maggiorini M, Wuthrich RP. Aliskiren-associated acute renal failure with hyperkalemia. Clin Nephrol. 2009;71(3)326–328.