Abstract
Background: Combination therapy with pegylated interferon (pegIFN)-α and ribavirin (RBV) for chronic hepatitis C virus (HCV) infection is associated with reduction in hemoglobin (Hb) concentrations and anemia. The aim of this study was to evaluate the magnitude and frequency of change in Hb and determine the predictive risk factors for Hb decrease during this therapy. Methods: We enrolled 308 patients with chronic HCV infection who were receiving weekly subcutaneous pegIFN injection in combination with body weight-based oral RBV for 24 weeks. Clinical and virological characteristics were used for studying the predictors of decrease in Hb. Results: The majority (95%) of patients showed reduction in Hb concentration of at least 1 g/dL during pegIFN and RBV combination therapy. The mean and median maximal decrease in Hb level of the study patients was 3.9 g/dL (range −0.3 to 8.2 g/dL; interquartile range 2.8–5.0 g/dL). Of all patients, 49.4% showed a reduction in Hb level of more than 4 g/dL; a higher number of male patients than female patients showed an Hb decrease of >4 g/dL. Multivariate analysis of our data showed that older age, high baseline Hb concentration, high HCV RNA viral load, low estimated glomerular filtration rate (eGFR), and low platelet count were independent predictors of significant decline in Hb levels. Conclusions: Patients with low eGFR before antiviral therapy may have an increased risk of RBV-related anemia and should be closely monitored. Clinician should consider the potential risk of significant reduction in Hb level according to eGFR while deciding the RBV dose.
INTRODUCTION
Chronic hepatitis C virus (HCV) infection, one of the most common causes of chronic liver disease, is a major global health problem and is associated with substantial morbidity and mortality from sequelae such as liver cirrhosis and hepatocellular carcinoma (HCC).Citation1–3 Approximately 180 million people worldwide are infected by HCV.Citation4,5 Twenty percent of all individuals with chronic hepatitis C eventually develop cirrhosis within approximately 20 years. It has been shown that 27% of HCV-infected people develop cirrhosis and 25% develop HCC.Citation6 Several previous studies have indicated that chronic HCV-infected patients can benefit from interferon (IFN)-based therapy, which reduces the risk of developing cirrhosis and HCC, particularly in responders,Citation7–10 and prevents the development of liver-related complications and improving survival.
Ribavirin (RBV), a guanosine nucleoside analog, exerts its antiviral effect through the inhibition of RNA-dependent RNA polymerase of HCV and induction of HCV RNA mutagenesis.Citation11 However, this drug can also deplete the adenosine triphosphate levels within red blood cell and cause defective antioxidant defense, which results in dose-dependent hemolytic anemia. IFN also contributes to the development of anemia by suppressing bone marrow function, which impairs the compensatory reticulocytosis occurring in response to RBV-induced hemolytic anemia.Citation12 Treatment of chronic HCV infection using a combination of RBV and the standard IFN has been shown to increase the sustained virological response (SVR) rate from 5–13% to 38–53%.Citation13–15 Further, treatment with a combination of RBV and pegylated interferon (pegIFN) has been shown to increase the SVR rate to 54–56% compared with treatment with pegIFN alone, which increased the SVR rate to 29%.Citation16,17
Fried et al.Citation16 showed that the hemoglobin (Hb) level dropped to its nadir after approximately 4 weeks of antiviral treatment. SulkowskiCitation18 showed that in chronic HCV patients treated with IFN and RBV, more than half the patients showed a reduced Hb level of >3.0 g/dL while undergoing the combination antiviral therapy. Another study showed a mean maximal decrease in Hb level of up to 4.0 g/dL during a 24-week IFN + RBV therapy.Citation19 The incidence of anemia during the pegIFN + RBV therapy was 11–12%.Citation20 By administering a body weight (BW)-based RBV dose, a better SVR could be achieved; however, this improvement was also associated with a higher rate of developing anemia. Treatment-related hemolytic anemia is an important side effect and frequently necessitates reduction of RBV dose. Because higher RBV concentration is associated with better SVR rate,Citation21 a reduction of RBV dose may decrease the antiviral effect and lead to increased risk of treatment failure.
Although several baseline factors were associated with increased risk of anemia development during antiviral therapy, most of the data obtained were from the IFN + RBV therapy. PegIFN with RBV combination therapy has been shown to improve therapeutic response and became the standard treatment for chronic HCV infection. However, only few studies have evaluated the predictors for the degree of Hb reduction and risk factors for Hb reduction with this treatment regimen.Citation22,23 One study showed that increased baseline creatinine (Cr) clearance was associated with decreased probability of Hb reduction to ≥2.5 g/dL, but it only enrolled patients with hepatitis C genotype 1. The aim of this study was to evaluate the severity and frequency of anemia among chronic HCV-infected patients with genotypes 1, 2, and 3 undergoing pegIFN + RBV therapy and the association of estimated glomerular filtration rate (eGFR) with the degree of Hb decline.
PATIENTS AND METHODS
Patients
This retrospective study was conducted at our institute between October 2003 and July 2009. We enrolled 308 patients who had chronic HCV infection, defined as seropositive for anti-HCV and HCV RNA for more than 6 months. All the patients had Cr levels below the upper limit of the normal range (male, 1.27 mg/dL; female, 1.03 mg/dL). None of the patients had received any antiviral therapy before enrollment. We excluded patients with concurrent hepatitis B virus (HBV) or human immunodeficiency virus (HIV) infection, toxic hepatitis, autoimmune hepatitis, primary biliary cirrhosis, Wilson’s disease, or hemoglobinopathies. Patients showing clinical or biochemical evidence of decompensated liver cirrhosis, chronic alcohol abuse, and psychiatric problems were also excluded. The Hb level of the enrolled patients was above 8.0 g/dL.
Methods
All 308 patients received weekly subcutaneous pegIFN injection plus daily oral RBV for 24 weeks. The prescribed types of pegIFNs were pegIFN-α-2a (180 μg) or weight-based pegIFN-α-2b (1.5 μg/kg). For genotype-1-HCV-infected patients with BW < 75 kg, the oral RBV dose was 1000 mg/day and for those with BW > 75 kg, 1200 mg/day. For genotype non-1-HCV-infected patients, the RBV dose was 800 mg/day. The follow-up schedule during the treatment course was weekly outpatient visit during the first 4 weeks of treatment, visits once in 2 weeks between the 5th and 12th weeks, and monthly visit during the last 12 weeks. Baseline demographic and clinical data, including age, gender, BW, body mass index (BMI), HCV viral load and genotype, complete blood count (CBC), and serum alanine aminotransferase level (ALT) and Cr concentrations were recorded. CBC and liver biochemical tests were conducted during each outpatient visit. All hematological, biochemical, and virological tests were performed at the clinical laboratories of Chang Gung Memorial Hospital. The study was performed in accordance with the ethical guidelines of the International Conference on Harmonization for Good Clinical Practice.
Anti-HCV tests were conducted using a third-generation enzyme immunoassay kit (AxSYM® HCV Version 3.0; Abbott Laboratories, Berkshire, UK). Serum HCV RNA was quantified using real-time polymerase chain reaction (PCR) system (COBAS® AmpliPrep Instrument and COBAS® TaqMan® 48 Analyzer; Roche Molecular Systems, Inc., Branchburg, NJ, USA) with a detection limit of 15 IU/mL. HCV genotype was determined using linear probe assay (VERSANT™ HCV Genotype Assay (LiPA); Bayer Corporation, Tarrytown, NY, USA). Routine liver biopsy was advised and performed after obtaining the patients’ consent. Advanced fibrosis was defined as a fibrosis score of ≥4 on the Ishak modified histology activity index (HAI) or a Metavir fibrosis score of ≥3. eGFR was calculated on the basis of the Cockcroft–Gault (CG) formula.
Statistical Analysis
Continuous demographic data were expressed as mean ± standard deviation (SD), and a 2-tailed Student’s unpaired t-test was performed to evaluate the differences among the mean values. Differences among groups of categorical variables were analyzed using a chi-square test or Fisher’s exact test. Significant baseline variables associated with Hb decline were identified by performing simple linear regression analysis. The significant factors were then subjected to multivariate analysis with a stepwise linear regression model to test the interactions between different significant covariates. The statistical analyses were performed using the SPSS ver. 12.0 software package (SPSS Inc., Chicago, IL, USA), and a p-value of <0.05 was considered statistically significant.
Table 1. Demographic and baseline characteristics of the study patients.
RESULTS
Patient Demography and Clinical Characteristics
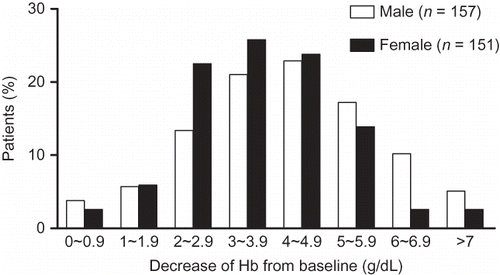
All 308 patients who underwent the pegIFN + RBV therapy fulfilled the criteria for analysis. Baseline characteristics of these patients are shown in . The mean age of patients was 54 years, and 51% were men. The genotypes of HCV included genotypes 1, 2, and 3; 57% patients were infected with HCV genotype 1. The mean serum HCV RNA before initiation of therapy was 5.76 × 106 copies/mL, and 67.5% patients had high HCV load. One hundred and fifty-two patients had biopsy-proven, advanced fibrosis before initiation of treatment. The mean eGFR at baseline was 89 mL/min. The mean Hb at baseline was 14.3 g/dL, with men having a higher Hb level than women (15.3 vs. 13.3 g/dL; p < 0.001). The mean and median maximal decrease in the Hb level of the study patients was 3.9 g/dL, with a range of −0.3 to 8.2 g/dL and an interquartile range of 2.8–5.0 g/dL. Although 49.4% of all patients showed an Hb decrease of more than 4 g/dL (), more male patients than female patients showed Hb decrease (>4 g/dL; 55.4% vs. 43%, respectively; p = 0.031). The mean decline in Hb concentration in male and female patients was 4.14 g/dL and 3.96 g/dL, respectively.
With regard to modification of treatment regimen, the majority of the patients with a decrease in Hb level of ≥3.9 g/dL had adjusted the dosage of the combination therapy (16% vs. 8%; p = 0.019). The rate of RBV dose reduction was greater in patients showing an Hb level reduction ≥3.9 g/dL than that in patients with less Hb change with a borderline significance (14% vs. 8%; p = 0.057).
Predictors for Hb Reduction During pegIFN and RBV Treatment
Simple linear regression analysis showed that a marked Hb reduction during pegIFN + RBV therapy could be predicted by increased age, male gender, presence of pathologically proven bridging fibrosis or cirrhosis, high pretreatment HCV RNA viral load, high Hb level, and low platelet count and eGFR at baseline (). Serum Cr level was not a predictor factor. By performing a multivariate analysis with a stepwise multiple regression model, we determined the independent predictive values of age, gender, histopathological stages of liver fibrosis, HCV genotype, HCV RNA levels, mean doses of RBV per kilogram of BW, GFR, Hb concentrations and platelet count before initiating the treatment, and BMI for the reduction in Hb level during therapy. The strongest predictor for the reduction in Hb levels during the antiviral treatment was high pretreatment HCV RNA viral load [B = 0.559 (95% CI: 0.210, 0.907)] followed by high pretreatment Hb levels [per 1 g/dL increase; B = 0.320 (95% CI: 0.215, 0.425)] and age [per 10-year increase; B = 0.303 (95% CI: 0.267, 0.539)] (). The platelet counts and eGFRs were also found to be significantly associated with Hb decline.
Table 2. Factors associated with hemoglobin decrease analyzed using simple linear regression analysis.
Table 3. Factors associated with hemoglobin decrease analyzed using stepwise multiple regression analysis.
DISCUSSION
To our knowledge, this is the first study to assess the Hb decline caused by pegIFN + RBV therapy—the current standard-of-care regimen for the treatment of patients with chronic HCV infection with all genotypes and its association with eGFR. Our study showed that more than 95% of the male and female patients receiving pegIFN + RBV therapy developed a decline in Hb levels of at least >1g/dL. The mean maximal decrease in Hb level of the study patients was 3.9 g/dL; 55% men and 43% women showed a decrease of >3.9 g/dL. Compared with the therapy using IFN or pegIFN monotherapy, the pegIFN + RBV therapy effected greater improvement in the SVR rate and decrease in the relapse rate after 6 months of follow-up.Citation16,17 However, treatment-related hemolytic anemia was one of the most important side effects that necessitated dose reduction or treatment discontinuation.Citation16,17,19,24,25 IFN or pegIFN, which suppresses bone marrow hematopoiesis, also inhibits compensatory erythropoiesis and causes prominent Hb decline. Because higher RBV concentration is associated with better SVR rate,Citation21,26 reducing the RBV dose in patients may decrease the antiviral effect. Compared to the study by Takaki et al.,Citation19 which showed a mean maximal Hb decrease of 4 g/dL during IFN + RBV therapy, the pegIFN + RBV in our study did not result in more severe treatment-related Hb decline. However, a substantially high proportion of patients with a decline in Hb of >3.9 g/dL had reduced their RBV doses than those with a decline in Hb of less than 3.9 g/dL.
Therefore, a better understanding of the baseline clinical factors significantly associated with decline in Hb level is required and may help clinicians in identifying patients at high risk of developing anemia during antiviral therapy. Our study showed that several baseline factors are predictive of absolute decrease in Hb level (). Snoeck et al.Citation22 reported that gender and RBV dose per kilogram of BW were the most important prognostic factors for the incidence of anemia, followed by baseline Hb levels, age, ALT, and presence of cirrhosis. Women were predicted to have a higher likelihood of decrease in Hb concentration (<10 g/dL) than men. However, in view of absolute Hb decrease, gender did not appear to be a predictive factor in our study. Our study showed that higher baseline Hb was significantly associated with increased decline in Hb during antiviral therapy. Although Snoeck et al.Citation22 reported that low baseline Hb value was a predictor of decline of Hb to below 10 g/dL, this could be because most patients (95%), as in our study, developed at least a 1 g/dL reduction in Hb level during treatment; hence, we assume that patients with lower baseline Hb level have more chance to have a decline of Hb level to less than 10 g/dL.
Because RBV and its metabolites, triazole carboxamide and triazole carboxylic acid, are eliminated by the kidney, the serum concentration of RBV could be altered by renal function. Bruchfeld et al.Citation27 demonstrated that RBV clearance was linearly dependent on renal function with a marginal nonrenal clearance, which in turn is dependent on the BW and age. eGFR was a significantly better predictor of RBV clearance than BW alone. Lindahl et al.Citation28 also showed that the RBV dose per kilogram of BW was not correlated with the RBV-induced decrease in Hb level. The concentration of RBV was related to the decrease in Hb level. Our findings confirm that a significant absolute decrease in Hb was independently associated with decreasing eGFR as estimated using the CG formula rather than with RBV dose per kilogram of BW as suggested by Snoeck et al.Citation22 And Cr alone is not a predictor of changes in Hb level in our study. Thus, it is useful for clinicians to calculate the eGFR before initiating pegIFN + RBV therapy to assess the risk of Hb decrease during the therapy, even though patients have normal serum Cr level.
In conclusion, our study showed that low eGFR, older age, high baseline Hb level, high HCV RNA viral load, and low platelet count were independent predictors for significant Hb decline during antiviral therapy. eGFR was found to be a valuable and early predictor for subsequent Hb decrease and may be used for identifying patients at high risk of developing anemia during therapy; thus, eGFR can be used for efficient clinical management of Hb decrease during therapy for HCV infection and for prevention of adverse effects. Clinicians should also consider the RBV dose and potential risk of significant Hb decrease according to the eGFR to minimize adverse events and enhance the antiviral effects of the drug.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13(17):2436–2441.
- Tsukuma H, Hiyama T, Tanaka S, . Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328(25):1797–1801.
- Fassio E. Hepatitis C and hepatocellular carcinoma. Ann Hepatol. 2010;9:119–122.
- Hadigan C, Kottilil S. Hepatitis C virus infection and coinfection with human immunodeficiency virus: Challenges and advancements in management. J Am Med Assoc. 2011;306(3):294–301.
- Ghany MG, Strader DB, Thomas DL, . Diagnosis, management, and treatment of hepatitis C: An update. Hepatology. 2009;49(4):1335–1374.
- Perz JF, Armstrong GL, Farrington LA, . The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–538.
- Huang JF, Yu ML, Lee CM, . Sustained virological response to interferon reduces cirrhosis in chronic hepatitis C: A 1386-patient study from Taiwan. Aliment Pharmacol Ther. 2007;25(9):1029–1037.
- Shiratori Y, Imazeki F, Moriyama M, . Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132(7):517–524.
- Yu ML, Lin SM, Chuang WL, . A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: A nationwide, multicentre study in Taiwan. Antivir Ther. 2006;11(8):985–994.
- Imai Y, Kawata S, Tamura S, . Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Osaka Hepatocellular Carcinoma Prevention Study Group. Ann Intern Med. 1998;129(2):94–99.
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436(7053):967–972.
- Kowdley KV. Hematologic side effects of interferon and ribavirin therapy. J Clin Gastroenterol. 2005;39(1 Suppl.):S3–S8.
- McHutchison JG, Gordon SC, Schiff ER, . Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339(21):1485–1492.
- Davis GL, Esteban-Mur R, Rustgi V, . Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339(21):1493–1499.
- Akram M, Idrees M, Zafar S, . Effects of host and virus related factors on interferon-alpha+ribavirin and pegylated-interferon+ribavirin treatment outcomes in chronic Hepatitis C patients. Virol J. 2011;8:234.
- Fried MW, Shiffman ML, Reddy KR, . Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982.
- Manns MP, McHutchison JG, Gordon SC, . Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358(9286):958–965.
- Sulkowski MS, Wasserman R, Brooks L, . Changes in hemoglobin during interferon alpha-2b plus ribavirin combination therapy for chronic hepatitis C virus infection. J Viral Hepat. 2004;11(3):243–250.
- Takaki S, Tsubota A, Hosaka T, . Factors contributing to ribavirin dose reduction due to anemia during interferon alfa2b and ribavirin combination therapy for chronic hepatitis C. J Gastroenterol. 2004;39(7):668–673.
- McHutchison JG, Manns MP, Brown Jr. RS, . Strategies for managing anemia in hepatitis C patients undergoing antiviral therapy. Am J Gastroenterol. 2007;102(4):880–889.
- Jen JF, Glue P, Gupta S, . Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther Drug Monit. 2000;22(5):555–565.
- Snoeck E, Wade JR, Duff F, . Predicting sustained virological response and anaemia in chronic hepatitis C patients treated with peginterferon alfa-2a (40KD) plus ribavirin. Br J Clin Pharmacol. 2006;62(6):699–709.
- Reau N, Hadziyannis SJ, Messinger D, . Early predictors of anemia in patients with hepatitis C genotype 1 treated with peginterferon alfa-2a (40KD) plus ribavirin. Am J Gastroenterol. 2008;103(8):1981–1988.
- Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 Suppl. 1):S237–S244.
- Hadziyannis SJ, Sette H Jr., Morgan TR, . Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140(5):346–355.
- Abstracts of the Biennial Meeting of the International Association for the Study of the Liver, April 15–16, 2002 and the 37th Annual Meeting of the European Association for the Study of the Liver, April 18–21, 2002. Madrid, Spain. J Hepatol. 2002;36(Suppl. 1):1–298 ; PubMed 12051238.
- Bruchfeld A, Lindahl K, Schvarcz R, . Dosage of ribavirin in patients with hepatitis C should be based on renal function: A population pharmacokinetic analysis. Ther Drug Monit. 2002;24(6):701–708.
- Lindahl K, Schvarcz R, Bruchfeld A, . Evidence that plasma concentration rather than dose per kilogram body weight predicts ribavirin-induced anaemia. J Viral Hepat. 2004;11(1):84–87.