Abstract
Hyperuricemia is particularly common in patients with arterial hypertension, metabolic syndrome, or kidney disease. Its role, however, as a risk factor for both renal and cardiovascular outcomes and in the context of the well-established interrelationship between cardiovascular disease and chronic kidney disease (CKD) is debated. For decades high serum uric acid levels were mainly considered the result of renal dysfunction and not a true mediator of renal disease development and progression. However, recent epidemiological studies suggest an independent association between asymptomatic hyperuricemia and increased risk of arterial hypertension, CKD, cardiovascular events, and mortality. Furthermore, data from experimental models of hyperuricemia have provided robust evidence in this direction. Hyperuricemia causes increased arterial pressure, proteinuria, renal dysfunction, and progressive renal and vascular disease in rats. The main pathophysiological mechanisms of these deleterious effects caused by uric acid are endothelial dysfunction, activation of local renin–angiotensin system, increased oxidative stress, and proinflammatory and proliferative actions. A small number of short-term, single-center clinical studies support the beneficial influence of pharmaceutical reduction of serum uric acid on total cardiovascular risk, as well as on renal disease development and progression. Hyperuricemia is probably related to the incidence of primary hypertension in children and adolescents, as serum uric acid lowering by allopurinol has an antihypertensive action in this group of patients. Finally, it is clear that adequately powered randomized controlled trials are urgently required to elucidate the role of uric acid in cardiovascular events and outcomes, as well as in the development and progression of CKD.
INTRODUCTION
Hyperuricemia is common in kidney disease due to decreased uric acid clearance. However, its role as a risk factor for kidney disease progression is largely disputed. It was primarily considered a marker of kidney damage and only secondarily an independent risk factor for kidney disease development and progression. Nevertheless, recent studies have raised the exciting possibility that uric acid may, indeed, have a contributory causal role in cardiovascular and renal diseases.Citation1
Following the discovery by Garrod in the early 1800s that elevated serum uric acid was the cause of gout, hyperuricemia was proposed to have a causal role in a variety of cardiovascular and renal conditions, including hypertension, arteriolosclerosis, kidney, and heart diseases.Citation1,2 In a paper published in 1879 that originally described essential hypertension, Frederick MahomedCitation3 noted that many of his subjects came from gouty families. Subsequently, he hypothesized that uric acid might be integral to the development of essential hypertension. Ten years later, this hypothesis reemerged when HaigCitation4 proposed low-purine diets as a means to prevent hypertension and vascular disease. In 1909, the French academician Henri HuchardCitation5 noted that renal arteriolosclerosis (the histologic lesion of hypertension) was present in subjects with hyperuricemia and suggested, for the first time, a potential association between uric acid and kidney damage.
Several, quite early, epidemiological observations support the association between hyperuricemia and renal disease. Indeed, as many as 20–60% of patients with gout had mild or moderate renal impairment, 25–50% had arterial hypertension, and 10–25% developed end-stage renal disease (ESRD) before uric acid-lowering agents became widely available.Citation6,7 The histologic lesion termed “gouty nephropathy,” especially prevalent in the past, consisted of glomerulosclerosis, interstitial fibrosis, and renal arteriolosclerosis, often with focal interstitial urate crystal deposition. These findings have been observed in autopsies of 79–99% of patients with gout.Citation8 Thus, since early twentieth century gout and, consequently, hyperuricemia have been considered significant risk factors for cardiovascular and renal diseases.
By the mid-twentieth century, however, the causal association between uric acid and cardiovascular disease has been questioned, as it was recognized that the association of gout with cardiovascular disease might simply reflect that both have similar risk factors, such as obesity, hypertension, metabolic syndrome, and kidney disease.Citation1,2 In addition, large epidemiological studies produced contradictory results, while many of them failed to demonstrate an independent association of hyperuricemia with cardiovascular disease. Simultaneously, many studies overemphasized the role of uric acid as an antioxidant. Moreover, hyperuricemia observed in chronic kidney disease (CKD) of any cause was considered secondary to a decrease in either glomerular filtration rate (GFR) or hyperinsulinemia of the metabolic syndrome.Citation2 Thus, hyperuricemia in CKD was considered a benign finding and uric acid was likely not a true cardiovascular or renal risk factor. A “requiem” for uric acid and its role in the pathogenesis and progression of kidney disease was celebrated in a review article published in Kidney International in 1986.Citation9 Consequently, uric acid measurement was removed from the panel of routine laboratory tests and was deleted from the list of risk factors provided by most cardiovascular and renal societies.
In the late 1990s certain observations led the specialists to reevaluate the significance of uric acid in cardiovascular and renal diseases.Citation10 The inconsistency and inconclusiveness of major studies have been attributed, at least in part, to the direct association between hyperuricemia and traditional cardiovascular risk factors. Thus, the potential role of hyperuricemia as an independent cardiovascular risk factor can hardly be evaluated in multivariate analysis models. However, uric acid can be a causal risk factor for cardiovascular disease without being simultaneously an independent risk factor. Similarly, the finding that a decreased GFR caused uric acid retention did not rule out the possibility that hyperuricemia might contribute to the subsequent progression of kidney disease. Finally, and perhaps more importantly, the conclusion that uric acid was not significant as a cardiovascular and renal risk factor had never been tested by direct experimental studies in animal or cell cultures.Citation1
Recent epidemiological, experimental, and clinical studies have consistently raised the exciting possibility that uric acid may indeed have a causative role in the onset and progression of hypertension, cardiovascular disease, and renal disease.Citation2 The purpose of this article is a critical presentation of the current state of knowledge on the role of uric acid in the highly interesting issue of kidney disease prognosis and progression.
BIOLOGICAL EFFECTS OF URIC ACID IN VITRO
Uric acid is the end product of purine metabolism in humans. Most mammals possess the enzyme uricase, which converts uric acid to the more soluble allantoin, which is freely excreted in the urine. This enzyme is absent in humans and, thus, uric acid remains the end product of the catabolic pathway. Previously, uric acid was considered a biologically inert substance, but then was found to have many biological properties that could be either beneficial or detrimental to humans.Citation11
A major beneficial property is its ability to act as an antioxidant, and possibly one of the most important antioxidants in plasma. Urate, the soluble form of uric acid in the blood, reacts with various oxidants, such as hydrogen peroxide, hydroxyl radicals, and nitric oxide (NO) derivatives, and neutralizes their toxic effect.Citation12 Furthermore, uric acid inhibits the degradation and deactivation of extracellular superoxide dismutase, an enzyme with significant antioxidant activity in vascular endothelium.Citation13 These beneficial effects of uric acid have led to the hypothesis that a rise in serum uric acid, which is common in patients with cardiovascular disease, might represent a compensatory mechanism to counteract the increased oxidative stress that occurs in these conditions.Citation14,15 However, this hypothesis is in total contradiction to the established association of hyperuricemia with increased incidence of cardiovascular events both in the general population and in patients at high cardiovascular risk.
Although uric acid is thought to have antioxidant activity in the extracellular space, once it enters cells, it seems to exert various deleterious effects, many of which have been documented in experimental studies.Citation2 The main pathophysiological mechanisms by which uric acid exerts its deleterious effects are summarized in .
Table 1. Major pathophysiological mechanisms of uric acid-induced damage.
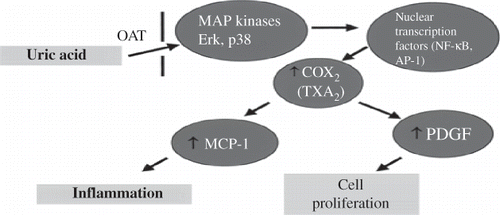
Uric acid enters vascular smooth muscle cells through specific organic anion transporters and activates intracellular protein kinases (p38 and Erk 1/2) and nuclear transcription factors [nuclear factor kappa B (NF-κB) and activator protein-1 (AP-1)], resulting in a proliferative and proinflammatory phenotype. Thus, vascular smooth muscle cells of blood vessels produce growth factors (platelet-derived growth factor), vasoconstrictive substances [angiotensin II (A2) and thromboxane (TXA2)], cytokines, and proinflammatory molecules [C-reactive protein (CRP) and monocyte chemoattractant protein-1 (MCP-1)].Citation16,17 At the same time, uric acid appears to induce increased expression (upregulation) of the type 1 angiotensin II receptor in endothelial cells and vascular smooth muscle cells.Citation2 Increased uric acid concentration in the blood stimulates the production of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α ), thereby promoting proinflammatory mechanisms.Citation11 These pathophysiological pathways by which uric acid exerts its proliferative and proinflammatory actions are shown schematically in .
Figure 1. Pathways by which uric acid induces proliferative and inflammatory mechanisms. Uric acid enters vascular smooth muscle cells through specific OAT and activates intracellular MAP kinases, p38 and Erk 1/2, and nuclear transcription factors (NF-κB and AP-1), leading these cells to acquire a proliferative and inflammatory phenotype. Thus, vascular smooth muscle cells, through increased expression of COX-2 and TXA2, produce cytokines, growth factors (PDGF), and proinflammatory molecules (MCP-1).
Note: OAT, organic anion transporter; MAP, mitogen-activated protein; NF-κB, nuclear factor kappa B; AP-1, activator protein-1; COX-2, cyclooxygenase-2; TXA2, thromboxane; PDGF, platelet-derived growth factor; MCP-1, monocyte chemoattractant protein-1.
In addition, uric acid inhibits proliferation and migration of endothelial cells and secretion of NO, contributing to endothelial dysfunction.Citation18 Uric acid infusion in the forearm of healthy subjects resulted in impaired acetylcholine-induced vasodilation, thereby further documenting impaired NO release.Citation19 Simultaneously, uric acid seems to trigger platelet adhesion and aggregation, thus favoring vascular thrombosis.Citation20 Finally, although uric acid is considered to exert antioxidant activity in plasma, its reaction with oxidizing agents appears to have harmful consequences associated with the release of active free radicals, especially when other antioxidants are at low concentrations.Citation2,11
EPIDEMIOLOGICAL DATA
It is generally accepted that hyperuricemia is particularly common in patients at high cardiovascular risk, such as those suffering from metabolic syndrome, hypertension, renal disease, or cardiovascular diseaseCitation11,21,22 Consistent with this observation has been the finding that elevated uric acid predicts the development of cardiovascular and renal diseases in the general population and in patients at high cardiovascular risk. Moreover, the presence of hyperuricemia is an adverse prognostic factor and is associated with increased morbidity and mortality.Citation10 Numerous recent and earlier epidemiological studies have demonstrated the association of hyperuricemia with cardiovascular events and mortality not only in the general population, but also in patients with hypertension or preexisting cardiovascular or renal diseases.Citation23–30 On the other hand, the Framingham Study and the Atherosclerosis Risk in Communities (ARIC) Study did not support such an association.Citation31,32 Furthermore, a recent analysis of the ARIC database demonstrated that although higher serum uric acid concentration is associated with mortality in the non-CKD population even after adjustment for metabolic syndrome, the presence of CKD attenuates this association.Citation33 The inconsistency of the data was confirmed by a recently published study in a large historical cohort of a national insurance provider that documented a stronger association between serum uric acid concentration and cardiovascular morbidity in patients with severely decreased GFR.Citation34 Thus, the challenge remains as to whether uric acid has a pathogenic role in the onset and progression of renal and cardiovascular diseases.
Uric Acid and Hypertension
The potential direct association between hyperuricemia and hypertension has attracted special scientific attention since the mid-twentieth century. Previously, this association was strongly questioned and only a single study had reported that hyperuricemia is an independent risk factor for the development of arterial hypertension.Citation35 However, it has been documented early enough that hyperuricemia (serum uric acid >6.5 mg/dL) is present in 25–40% of untreated hypertensive subjects, in 50% of those taking diuretics, and in at least 75% of those with malignant hypertension.Citation36 These numbers increase dramatically when serum uric acid in the high normal range is included. Indeed, a linear relationship has been found between uric acid blood levels and systolic blood pressure (BP) in both white and black individuals.Citation37 In certain special cases of hypertension, such as cyclosporine-associated hypertension and preeclampsia, the correlation between elevated serum uric acid and hypertension exceeds 70%.Citation38
In the period after 1990, several large epidemiological studies have been published that support the role of uric acid as an independent risk factor for the development of arterial hypertension.Citation23,25,26,28–30 Consequently, the skepticism over a potential uric acid and high BP association, prevalent in the previous decades, has changed significantly. For example, in 3300 individuals with normal BP and renal function and without known heart disease participating in the Framingham Heart Study, an independent correlation was found between serum uric acid levels and the development of arterial hypertension over a 4-year follow-up period.Citation30 The association between hyperuricemia and hypertension was repeatedly observed in the 1950s and through the 1980s it received relatively little attention mainly due to the lack of an exact causal mechanism. Thus, mild elevations of serum uric acid were ignored in medical practice and uric acid was not considered a risk factor for hypertension by the most distinguished scientific societies (American Heart Association, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure).Citation39,40
Both experimental and clinical data show that the relationship between serum uric acid and BP is stronger in younger subjects with some dampening over time.Citation41,42 A recent prospective case–control study in men of different ages (mean age of 61 years, range 47–81), who participated in the large Health Professionals’ Follow-Up Study, found no independent association between the uric acid level and the risk for incident hypertension among older men (>60 years). Although a statistically significant correlation was observed in younger men, this correlation was attenuated and became nonsignificant after adjustment for metabolic factors, such as cholesterol, triglycerides, and fasting insulin.Citation42 Any relationship between uric acid and hypertension seems to be less important with advancing age, when atherosclerosis evidenced by aortic stiffening of the aorta is prevalent. Furthermore, increasing age is associated with irreversible vascular damage and significant renal injury both resulting in mainly salt-sensitive and uric acid-independent hypertension.Citation43
The evaluation of the potential correlation between uric acid levels and hypertension has focused recently on children and adolescents. This was initially considered odd, as hypertension is, undoubtedly, a disease of middle and advanced age. In children, however, the absence of comorbidities allows an evaluation of the potential causal relationship between hypertension and hyperuricemia without the interference of various confounding factors that are prevalent in adults.Citation44 Thus, studies of new-onset essential hypertension in adolescents have reported an elevation of uric acid >5.5 mg/dL in almost 90% of hypertensive subjects versus 0% of controls, and the relationship of uric acid with hypertension was linear and dramatic.Citation43 In addition, a very recent study in 63 pediatric hemodialysis patients found a positive association between serum uric acid and pretreatment BP independent of volume, nutritional, and weight status.Citation45
Uric Acid in CKD Onset and Progression
Several observational studies have examined whether uric acid is an independent risk factor for the development and progression of CKD, leading to inconclusive and, often, conflicting results. As already mentioned, 20–60% of patients with gout and hyperuricemia developed renal impairment, accompanied mainly by histological lesions of glomerulosclerosis, interstitial fibrosis, arteriolosclerosis, and often focal deposition of urate crystals in the outer medulla.Citation6 Elevated levels of uric acid have been reported to predict the development of renal insufficiency in individuals with normal renal functionCitation9 and correlated with the onset of proteinuriaCitation46 and renal dysfunctionCitation47 in patients with type II diabetes mellitus. Interestingly, uric acid at diagnosis is an independent predictor for renal disease progression in patients with immunoglobulin A (IgA) nephropathy, although a causal relationship cannot be established by such studies.Citation48,49
Several studies of clinical epidemiology have been published over the last decade and, although with significant limitations, they consistently reveal a modest but statistically significant association between hyperuricemia and the development of CKD and even ESRD. A Japanese study of 6403 individuals, residents of Okinawa City, with normal renal function assessed the significance of hyperuricemia on the early detection of renal dysfunction and found baseline serum uric acid significantly correlated with the rise of serum creatinine in both men and women over a 2-year follow-up period.Citation50 Specifically, serum uric acid ≥8.0 mg/dL is associated with greater risk (by 2.9 times in men and by 10.4 times in women) for developing high serum creatinine, after adjusting for various confounding factors. However, baseline uric acid levels showed no correlation with a change in creatinine clearance as calculated by the Cockcroft–Gault equation, and the authors provided no explanation for this discrepancy. The same research group in a subsequent study investigated the significance of hyperuricemia as a risk factor for ESRD in 48,177 people of the general population older than 20 years.Citation51 It was demonstrated that hyperuricemia is an independent predictor of ESRD in women over a 7-year follow-up period, while in men the difference was not statistically significant. The authors concluded that strategies to control serum uric acid levels in the normal range may reduce the incidence and the burden of ESRD.
It should be noted that several of the earlier studies did not use adequate models of multivariate analysis and therefore could not exclude all possible confounding factors that may interfere with the relationship between uric acid and CKD.Citation52 But apart from any methodological problems, the results of epidemiological studies on the question whether uric acid is an independent risk factor for the onset and progression of kidney disease are often contradictory. Thus, during a long-term follow-up of 25 years, the large Multiple Risk Factor Intervention Trial Study revealed that high blood levels of uric acid were associated with the progression to ESRD.Citation53 However, correlation was attenuated when individuals with baseline Modification of Diet in Renal Disease (MDRD)Citation54 estimated GFR (eGFR MDRD) below 60 mL/min/1.73 m2 or with proteinuria were excluded, suggesting that uric acid is rather an indicator of reduced renal function and not a true mediator of renal function decline.
The correlation of uric acid with kidney disease progression was further investigated in a recent prospective study of individuals aged 65 years and older who participated in a large study of the general population, the Cardiovascular Health Study (CHS).Citation55 Kidney disease progression was defined as a decrease in eGFR MDRD of 3 mL/min/1.73 m2 per year or greater or an incident CKD (eGFR <60 mL/min/1.73 m2). Uric acid levels at baseline were associated strongly and linearly with prevalent CKD (eGFR <60 mL/min/1.73 m2), an association that remained statistically significant after adjustment for multiple confounders. A 14% increase in kidney disease progression was demonstrated per 1 mg/dL rise in uric acid. However, no association was documented between baseline uric acid levels and incident CKD, suggesting that uric acid is not a risk factor for CKD development. A weak association was found between baseline uric acid levels >5.9 mg/dL and subsequent decrease in eGFR MDRD, while this association disappeared with Cockcroft–Gault eGFR. A main limitation of this study, as stated by the authors, was the lack of information on albuminuria, which is now an established risk factor for kidney disease progression.
An interesting epidemiological study, published recently, evaluated data on a large population sample with normal renal function in two community-based cohorts, the ARIC and the CHS , and investigated the relationship between uric acid at baseline and incident CKD over 9 years of follow-up.Citation56 It was found that baseline uric acid levels were associated with increased risk for incident CKD with odds ratios increasing with the rise in uric acid levels when renal function was assessed by eGFR MDRD or simple serum creatinine, and after adjusting for various confounding factors. The authors concluded that an elevated uric acid level is an independent risk factor for the development of CKD in the general population. The sensitivity analysis of this study revealed that a substantial part of the relationship between uric acid and renal disease was mediated by systemic hypertension. Data on baseline proteinuria and allopurinol use were not available.
The role of uric acid as an independent risk factor for the development of new-onset CKD has been confirmed in a second recent epidemiological study in 21,475 healthy Austrian volunteers participating in the prospective study Vienna Health Screening Project with a median follow-up of 7 years.Citation57 The study participants were classified according to the serum uric acid levels into three groups: uric acid levels <7 mg/dL, moderate hyperuricemia (7–8.9 mg/dL), and severe hyperuricemia (≥9 mg/dL). Incident CKD was defined as eGFR MDRD <60 mL/min/1.73 m2. After adjusting for various confounding factors, including the level of kidney function (eGFR) at baseline, uric acid was independently associated with new-onset CKD in both men and women and in groups with moderate and severe hyperuricemia. Of note, the risk for CKD was higher in those with elevated BP, while proteinuria did not appear to affect the relationship between uric acid and incident CKD. Similar results have been demonstrated in the very recently published Jerusalem Lipid Research Clinic cohort study among 2449 participants with normal baseline kidney function and 24–28 years of follow-up.Citation58 Serum uric acid predicted the incidence of acute and chronic renal insufficiency and all-cause mortality independently of baseline kidney function and covariates.
The potential pathogenic role of baseline uric acid in the progression of already established renal disease remains as yet an area of great uncertainty. Thus, when the MDRD study (N = 840) database was examined, uric acid was not a predictor of renal progression, defined as the requirement for dialysis or transplantation, in patients with stages 3 to 4 CKD during a median follow-up of 10 years.Citation59 The results of this study were unchanged by adjustment for BP, suggesting that the increased risk was independent of a relationship between uric acid level and BP. Along the same lines, Sturm et al.Citation60 found in 227 nondiabetic patients with mild-to-moderate CKD that uric acid levels did not predict CKD progression, defined as doubling of baseline serum creatinine and/or terminal renal failure, during a 7-year follow-up period and after adjustment for baseline kidney function.
After the presentation of the main clinical epidemiology studies, the issue of data interpretation emerges. First, an independent risk factor is not necessarily causal. This may occur when the true causal risk factor is not included in the multivariate analysis. Likewise, a risk factor could still be causal without being independent. For example, assuming that uric acid promotes kidney disease progression not directly but through increased BP, the potential independent association between uric acid and renal disease progression is unlikely to be demonstrated if both hypertension and uric acid are considered in the multivariate analysis.Citation61 In addition, elevated serum uric acid is strongly associated with both prevalent kidney disease and CKD risk factors, including the metabolic syndrome, likely reflecting both renal handling of uric acid and collinearity among uric acid, obesity, hypertension, diabetes, and other lifestyle characteristics. Therefore, uric acid may be a marker of the severity of other risk factors rather than a direct contributor to kidney injury. In any case, it is difficult to evaluate the pathogenic role of uric acid in the development and progression of CKD, especially by using epidemiological studies. Although the latter could reveal valuable insights into pathogenic mechanisms, they cannot establish causality. To this direction, useful information could be provided from both experimental animal studies and interventional human trials.
URIC ACID AS A MEDIATOR OF RENAL DISEASE
Experimental Evidence
In 2001 the group of Richard Johnson developed an experimental model of hyperuricemia in rats, and the studies based on this had a major contribution to the revival of interest in the role of uric acid in hypertension and renal disease.Citation41 Most mammals have a low serum uric acid due to the action of uricase. In humans the uricase gene has undergone mutation and the enzyme has become nonfunctional. Low doses of oxonic acid (OA), an uricase inhibitor, caused mild hyperuricemia in rats without intrarenal deposition of urate crystals. Specifically, rats fed on OA developed uric acid levels on the order of 2.7–4.0 mg/dL, while, prior to OA, these rats had serum uric acid ranging from 0.5 to 1.4 mg/dL. Previous models of uricase inhibition (knockout rat’s uricase gene) led to massive uricosuria resulting in obstructive nephropathy and death within 3 months.Citation62
In this experimental model, hyperuricemic rats showed hypertension, afferent arteriole arteriolopathy, mild interstitial damage with mild striped interstitial fibrosis, glomerular hypertrophy, glomerulosclerosis, and albuminuria without intrarenal deposition of urate crystals, while the control group maintained normal BP.Citation41,63 Indeed, the rise in BP induced by hyperuricemia was higher in rats that were on a low-sodium diet compared with those that ingested normal amounts of sodium. The hypertension was associated, initially, with increased renin and decreased neuronal nitric oxide synthase (NOS1) in the juxtaglomerular apparatus. In the next stage, the hypertension was maintained by the hyperuricemia-induced vascular injury in the afferent arteriole.Citation41 The occurrence of both hypertension and tubulointerstitial injury was prevented when OA was coadministered with allopurinol (a xanthine oxidase inhibitor) or benziodarone (uricosuric drug). Additionally, in rats with established hyperuricemia following OA administration, the reduction of uric acid either through allopurinol addition or through OA discontinuation resulted in a decrease in BP and inhibited the development of tubulointestinal injury.Citation41,63 Finally, the involved mechanisms were further clarified by the observation that the coadministration of OA and enalapril (inhibition of angiotensin II synthesis) or l-arginine (stimulation of NO synthesis) resulted in a smaller increase in BP and less severe kidney damage as compared with the group of animals that received OA alone. Similarly, the coadministration of OA and allopurinol prevented increased renin expression in the juxtaglomerular apparatus and partially inhibited the reduction of nitrates in plasma observed in hyperuricemia rats.Citation41
Interestingly, hyperuricemic rats were also shown to have salt sensitivity in this experimental model, that is, a greater increase in BP for the same sodium load compared with normal rats.Citation64 A possible explanation for the mechanism has been provided in other experimental models that have shown that salt sensitivity may result from preglomerular vascular disease.Citation65 Thus, hyperuricemic rats showed, along with salt sensitivity, thickening and hypercellularity of the afferent arteriole of the glomerulus that resulted in a reduction in lumen diameter. This damage was similar to the pathognomonic lesion of essential hypertension in humans and was accompanied, as expected, by mild inflammation and tubulointerstitial fibrosis.Citation64 The vascular lesion in the afferent arteriole was independent of BP changes and was mediated in part by the direct effects of uric acid to induce vascular smooth muscle cells proliferation and also by activation of the renin–angiotensin–aldosterone system (RAAS).Citation66 Indeed, it was observed that, after OA discontinuation and normalization of uric acid levels, the damage in the afferent arteriole and the interstitial tissue as well as the resulting salt sensitivity persisted.Citation64 Subsequent experimental studies showed that hyperuricemia-induced vascular damage in the afferent arteriole impaired renal autoregulation resulting in renal vasoconstriction and systemic and glomerular hypertension.Citation67
To investigate the role of uric acid in renal disease progression, the classic model of renal progression induced by 5/6 nephrectomy (remnant kidney model) was used.Citation17 The rats were fed on OA for 6 weeks after 5/6 remnant kidney surgery with or without allopurinol or benziodarone. The major novel finding was that modest hyperuricemia markedly exacerbated renal progression, resulting in higher BP, greater proteinuria, progressive renal disease, and severe histological lesions (glomerulosclerosis, interstitial fibrosis). In addition, vascular lesions (thickening of afferent arterioles) were not solely a consequence of an elevation in systemic BP, as evidenced by more prominent vascular changes in preglomerular vessels of hyperuremic remnant kidney rats compared with historic controls with similar BP but significantly lower uric acid levels. Allopurinol significantly reduced uric acid levels and blocked the renal functional and histologic changes, while benziodarone reduced uric acid levels less effectively and only partially improved BP and renal function, with minimal effect on the vascular changes.Citation17 This study also showed that hyperuricemia was associated with increased renin expression in the renal cortex and cyclooxygenase (COX-2) expression in afferent arteriolar wall. Uric acid appeared to stimulate the vascular smooth muscle cells proliferation through the COX-2.
In another model of renal disease progression, that of cyclosporine-induced nephropathy, rats treated with cyclosporine and OA had higher uric acid levels in association with more severe histological lesions of cyclosporine-induced nephropathy (arteriolar hyalinosis, tubular injury, and striped interstitial fibrosis) compared with a group of animals treated with cyclosporine alone.Citation68 The mechanisms did not involve intrarenal urate crystal deposition and appeared to involve activation of RAAS and inhibition of intrarenal NO production.
Finally, recent experimental studies support the association between hyperuricemia and metabolic syndrome. Specifically, elevated uric acid levels were found to have causal role in the metabolic syndrome that was induced in rats fed on fructose. Fructose rapidly raises serum uric acid in blood and the resultant hyperuricemia might contribute to the onset of insulin resistance through, at least in part, the inhibition of NO.Citation69 Subsequently, it was observed that reducing uric acid levels in these rats resulted in improvement on most parameters of metabolic syndrome, such as hyperinsulinemia, hypertriglyceridemia, hypertension, and weight gain.Citation70 Although relevant clinical studies are still lacking, the above observations suggest that uric acid is involved in the pathogenesis of the metabolic syndrome that has become endemic throughout the developed countries in the past few decades.Citation71
Summarizing the conclusions of the important experimental studies mentioned above, hyperuricemia appears, for the first time, to have a pathogenetic role in both the occurrence of arterial hypertension and the progression of kidney disease. Additionally, these studies contribute significantly to the elucidation of the potential mechanisms through which the detrimental effects of uric acid are exerted. However, we must be very careful in extrapolating these findings from animals to humans. As already presented in detail, there are currently large epidemiological studies that point to the role of uric acid as an independent risk factor for the development and progression of kidney disease, but without definitive conclusions. Clinical interventional trials to clarify whether lowering of uric acid results in inhibition or, at least, slowing of renal disease progression are necessary.
Clinical Evidence
The most appropriate method to evaluate the involvement of uric acid in the pathogenesis of CKD progression is to determine whether lowering uric acid slows the rate of renal disease progression.Citation61 These clinical studies are very few and have several limitations. Some indirect evidence supporting the role of uric acid in the progression of kidney disease comes from the renal function deterioration observed in hyperucemic patients after allopurinol cessation.Citation72,73
It is worth noting at this point that allopurinol, by inhibiting xanthine oxidase, exerts antioxidant activity along with reducing serum uric acid levels.Citation2,74 Xanthine oxidase is a superoxide-producing enzyme and its activity can be inhibited completely by the use of allopurinol. In experimental models, allopurinol prevented vascular complications through its antioxidant effects by blocking the generation of free radicals.Citation2,74 However, a randomized study failed to demonstrate the superiority of allopurinol compared with placebo in reducing lipid peroxidation and total oxidative stress in patients with diabetes.Citation75 Therefore, whether allopurinol manifests its beneficial effects through its antioxidant properties is still not conclusive.Citation74
Additionally, allopurinol has consistently been found to improve endothelial function in diabetics, smokers, hypercholesterolemic patients, and those with congestive heart failure.Citation76 An important randomized, placebo-controlled trial on the effects of allopurinol on endothelial dysfunction and left ventricular hypertrophy in patients with stage 3 CKD has been recently published.Citation77 Specifically, 67 subjects were randomly assigned to allopurinol at 300 mg/day or placebo for 9 months, out of which 53 patients completed the study. Allopurinol significantly improved endothelial function, as indicated by flow-mediated dilation of the brachial artery, and reduced left ventricular hypertrophy, as evaluated by cardiac magnetic resonance imaging. Thus, this study demonstrated that allopurinol can regress left ventricular mass and improve endothelial function among patients with CKD. Both outcomes are associated with prognosis and may have important clinical implications. Indeed, this is the first study that showed a beneficial effect of allopurinol on left ventricular hypertrophy in humans. However, whether these effects translate into improvements in hard clinical endpoints is yet to be tested.
It is clear that the data mentioned above concern the potential role of uric acid lowering in reducing overall cardiovascular risk, rather than its direct effect on kidney disease progression. Similar results emerged from the LIFE (Losartan Intervention for Endpoint Reduction) Study. Although it was designed to compare losartan and atenolol in terms of cardiovascular morbidity and mortality, and not to investigate the role of uric acid reduction in cardiovascular events, the analysis of data showed that the hypouricemic effect of losartan appeared to explain a significant 29% of the favorable treatment effect on the primary composite endpoint (myocardial infarction, stroke, death).Citation78 Furthermore, the relationship between uric acid lowering by losartan and renal endpoints, defined as doubling of serum creatinine or ESRD, was determined in a more recent post hoc analysis of patients with type II diabetes and nephropathy participating in the Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan.Citation79 The risk of renal events was decreased by 6% per 0.5 mg/dL decrement in serum uric acid during the first 6 months of the study. This effect was independent of other risk markers, including eGFR and albuminuria. Adjustment of the overall treatment effects for serum uric acid attenuated losartan’s renoprotective effect from 22% to 17%, suggesting that approximately one-fifth of losartan’s renoprotective effect could be attributed to its effect on serum uric acid levels.
The direct clinical relevance of hypouricemic treatment in kidney disease progression was investigated in a prospective, randomized study where a group of patients with hyperuricemia and CKD were treated with allopurinol or continued their usual therapy for 12 months.Citation74 Study endpoints were an increase in serum creatinine greater than 40% of baseline values or ESRD and initiation of dialysis therapy. In the treatment group, only 16% of the patients reached endpoints during 12 months of follow-up as compared with 46% of the controls. Of note, there were no statistically significant differences in systolic or diastolic BP between the two groups at the end of the study despite a trend, not statistically significant, toward a lower serum creatinine in the treatment group compared with controls after 12 months of therapy. This pioneering study has several limitations that prevent generalization of the results, such as small sample size, short duration of treatment, and the Asian origin of patients. Moreover, during the study, patients continued to take antihypertensive drugs with potential renoprotective properties.
Likewise, Goicoechea et al.Citation80 studied patients with eGFR <60 mL/min who were randomly assigned to treatment with allopurinol or to continue the usual therapy and concluded after 24 months that allopurinol slows the progression of renal disease and reduces cardiovascular risk. Specifically, serum uric acid and CRP levels were significantly decreased in subjects treated with allopurinol and eGFR increased compared with significant decrease in controls. Allopurinol treatment slowed down renal disease progression independently of age, gender, diabetes, CRP, albuminuria, and renin–angiotensin system blockers use. After a mean follow-up time of 23.4 ± 7.8 months, it was demonstrated that allopurinol treatment reduces risk of new cardiovascular events compared with standard therapy. Despite these favorable results, further clinical trials are necessary to elucidate the role of uric acid lowering in renal disease progression. Only then, hypouricemic medical treatment could be widely implemented in daily clinical practice, especially since allopurinol treatment may be complicated by significant blood and kidney toxicity and even the potentially fatal Stevens–Johnson syndrome.Citation81
The association between hyperuricemia and essential hypertension in children and adolescents, according to epidemiological data, has been already presented in detail.Citation44 However, clinical trials are needed to establish this association. An interesting randomized, placebo-controlled, crossover clinical study investigated the effect of allopurinol on BP in adolescents with newly diagnosed, never-treated, stage 1 essential hypertension and serum uric acid levels ≥6 mg/dL who received allopurinol with a significant reduction in systolic and diastolic BP.Citation82 Indeed, two-third of the patients achieved normal BP while taking allopurinol versus only one patient in the placebo group. Therefore, allopurinol appears to reduce BP in adolescents with stage 1 essential hypertension and hyperuricemia. However, these preliminary findings require confirmation in larger clinical trials before hypouricemic medications can take their place in the treatment of essential hypertension in children and/or adults.
Hyperuricemia was shown to be associated with kidney disease progression after solid organ transplantation. It occurs with increased frequency in transplant recipients, mainly as a side effect of calcineurin inhibitors.Citation2 As already mentioned, hyperuricemia results in deterioration of cyclosporine-induced nephropathy in rats, while allopurinol administration appears to have renoprotective effect.Citation68 Indeed, the histological lesions found in experimental models of hyperuricemia are similar to those observed in chronic allograft nephropathy.Citation17 In the clinical setting, pharmaceutical reduction of serum uric acid in liver transplant recipients resulted in improved renal function.Citation83 Additionally, a recent case–control study showed that hyperuricemia aggravated cyclosporine-induced nephropathy in kidney transplant recipients.Citation84 Another retrospective clinical study involving renal transplant recipients, who were followed up for a minimum of 4 years, found that hyperuricemia was independently associated with the development of chronic allograft nephropathy and the incidence of cardiovascular events at 6 months after transplantation, even after adjustment for potential confounding factors.Citation85 It is, therefore, reasonable to assume that hyperuricemia is included in the nonimmunological risk factors for the development of chronic allograft nephropathy.Citation2
A possible pathogenetic role of hyperuricemia in acute renal failure irrespective of cause and beyond the well-known “gouty nephropathy” has recently been suggested.Citation86 Mechanisms by which uric acid may contribute to acute renal failure are renal vasoconstriction, proinflammatory and oxidizing effects of uric acid, microvascular injury, and impaired renal autoregulation. Of note, hyperuricemia may, in part, account for the paradoxical lack of benefit of diuretics in the management of acute renal failure. However, there are no clinical studies to date confirming the role of hyperuricemia in the pathogenesis of acute renal failure.
CONCLUSIONS
Uric acid, the end product of purine metabolism in humans, has diverse biological properties. Although it appears to act as an antioxidant in the extracellular space, once it enters cells, it seems to exert various deleterious effects, many of which have been documented in experimental studies. The major pathophysiological mechanisms of uric acid-induced damage are endothelial dysfunction, activation of local RAAS, increased oxidative stress, and proinflammatory and proliferative effects.
During the last decade, the role of uric acid as a cardiovascular risk factor as well as its possible correlation with renal disease progression have been reevaluated. Numerous earlier and recent epidemiological studies have demonstrated the independent association of hyperuricemia with arterial hypertension, cardiovascular events, and mortality in the general population, but also in patients at high cardiovascular risk. Fewer epidemiological studies support the causal association between hyperuricemia and renal disease development and progression, often with conflicting and inconclusive results. However, the medical community seems currently to be moving away from the assumption that hyperuricemia is only an indicator of renal dysfunction, reflecting reduced renal excretion. Uric acid is considered nowadays as a culprit and not only as an innocent bystander in hypertension and progression of renal disease.
Experimental studies confirmed the pathogenetic role of hyperuricemia in the onset and progression of hypertension and renal disease and also provided useful insights on the pathophysiological mechanisms through which the detrimental effects of uric acid are exerted. The vascular lesion in the glomerular afferent arteriole, observed in the experimental models of hyperuricemia, resembles the pathognomonic lesion of essential hypertension in humans and is accompanied by salt sensitivity and impaired renal autoregulation, resulting in systemic and glomerular hypertension.
Few clinical studies advocate the favorable effect of pharmaceutical uric acid reduction on cardiovascular risk, as well as on the onset and progression of renal disease. Hyperuricemia appears to be associated with the manifestation of essential hypertension in children and adolescents, as allopurinol demonstrated antihypertensive effect in this group of patients. Finally, it is clear that larger interventional clinical trials are needed to elucidate the role of uric acid in the genesis and progression of renal disease and to establish whether uric acid represents a remediable target for intervention in the context of primary prevention and inhibition of renal and cardiovascular disease progression.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Davies Jr NS. The cardiovascular and renal relations and manifestations of gout. J Am Med Assoc. 1897;29:261.
- Nakagawa T, Kang DH, Feig D, . Unearthing uric acid: An ancient factor with recently found significance in renal and cardiovascular disease. Kidney Int. 2006;69:1722–1725.
- Mahomed FA. On chronic Bright’s disease and its essential symptoms. Lancet. 1879;1:399–401.
- Haig A. On uric acid and arterial tension. BMJ. 1889;1:288–291.
- Huchard H. Arteriolosclerosis: Including its cardiac form. J Am Med Assoc. 1909;53:1129–1132.
- Berger L, Yü T. Renal function in gout: An analysis of 524 gouty subjects including long-term follow-up studies. Am J Med. 1975;59:605–613.
- Talbot JH, Terplan KL. The kidney in gout. Medicine. 1960;39:405–467.
- Gonick HC, Rubini MD, Gleason IO, Sommers SC. The renal lesion in gout. Ann Int Med. 1965;62:667–674.
- Beck L. Requiem for gouty nephropathy. Kidney Int. 1986;30:280–287.
- Johnson RJ, Kivlighn SD, Kim Y-G, Suga S, Fogo AB. Reappraisal of the pathogenesis and consequences of hyperuricemia in hypertension, cardiovascular and renal disease. Am J Kidney Dis. 1999;33:225–234.
- Johnson RJ, Kang DH, Feig D, . Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? J Am Soc Nephrol. 2003;41:1183–1190.
- Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: Implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–354.
- Hink HU, Santanam N, Dikalov S, . Peroxidase properties of extracellular superoxide dismutase: Role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol. 2002;22:1402–1408.
- Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: A reaction to atherosclerosis? Atherosclerosis. 2000;148:131–139.
- Filiopoulos V, Hadjiyannakos D, Metaxaki P, . Inflammation and oxidative stress in patients on hemodiafiltration. Am J Nephrol. 2008;28(6):949–957.
- Nakagawa T, Mazzali M, Kang DH, Sanchez-Lozada LG, Herrara-Acosta J, Johnson RJ. Uric acid: A uremic toxin? Blood Purif. 2006;24:67–70.
- Kang DH, Nakagawa T, Feng L, . A role of uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897.
- Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid induced C-reactive protein (CRP) expression: Implication on cell proliferation and nitric oxide production in human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562.
- Waring WS, Webb DJ, Maxwell SRJ. Effect of local hyperuricemia on endothelial function in the human forearm vascular bed. Br J Clin Pharmacol. 2000;49:511.
- Glinsberg MH, Kozin F, O’Malley M, McCarty DJ. Release of platelet constituents by monosodium urate crystals. J Clin Invest. 1997;60:999–1007.
- Feig D, Mazzali M, Kang DH, . Serum uric acid: A risk factor and a target for treatment? J Am Soc Nephrol. 2006;17:69–73.
- Feig D, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Eng J Med. 2008;359:1811–1821.
- Jossa F, Farinaro E, Panico S, . Serum uric acid and hypertension. The Olivetti heart study. J Hum Hypertens. 1994;8:677–681.
- Fang J, Alderman MH. Serum uric acid and cardiovascular mortality: The NHANES I epidemiologic follow-up study, 1971–1992. J Am Med Assoc. 2000;283:2404–2410.
- Taniguchi Y, Hayashi T, Tsumura K, Endo G, Fujii S, Okada K. Serum uric acid and the risk for hypertension and type 2 diabetes in Japanese men. The Osaka Health Survey. J Hypertens. 2001;19:1209–1215.
- De Leeuw PW, Thijs L, Birkenhäger WH, . Prognostic significance of renal function in elderly patients with isolated systolic hypertension: Results from the Syst-Eur Trial. J Am Soc Nephrol. 2002;13:2213–2222.
- Bickel C, Rupprecht HJ, Blankenberg S, . Serum uric acid as an independent predictor of mortality in patients with angiographically proven coronary artery disease. Am J Cardiol. 2002;89:12–17.
- Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480.
- Nakanishi N, Okamato M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and the risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–530.
- Sundstrom J, Sullivan L, D’Agostino R, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence in the Framingham Heart Study. Hypertension. 2005;45:28–33.
- Culleton BF, Larson MG, Kannel WB, . Serum uric acid and risk for cardiovascular and death: The Framingham Heart Study. Ann Intern Med. 1999;131:7–13.
- Moriarity JT, Folsom AR, Iribarren C, Nieto FJ, Rosamond WE. Serum uric acid and risk of coronary heart disease: Atherosclerosis risk in communities (ARIC) Study. Ann Epidemiol. 2000;10:136–143.
- Navaneethan SD, Beddhu S. Associations of uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol Dial Transplant. 2009;24:1260–1266.
- Neri L, Rocca Rey LA, Lentine KL, . Joint association of hyperuricemia and reduced GFR on cardiovascular morbidity: A historical cohort study based on laboratory and claims data from a national insurance provider. Am J Kidney Dis. 2011;58:398–408.
- Kahn HA, Medalie JH, Neufeld HN, Riss E, Goldbourt U. The incidence of hypertension and associated factors: The Israel ischemic heart study. Am Heart J. 1972;84:171–182.
- Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. N Engl J Med. 1966;275:457–464.
- Klein R, Klein BE, Cornoni JC, Maready J, Cassel JC, Tyroler HA. Serum uric acid. Its relationship to coronary heart disease risk factors and cardiovascular disease, Evans County, Georgia. Arch Intern Med. 1973;132:401–410.
- Curtis JJ, Luke RG, Jones P, Diethelm AG. Hypertension in cyclosporine-treated renal transplant recipients is sodium dependent. Am J Med. 1988;85:134–138.
- Pearson T, Blair S, Daniels S, . AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: Consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–391.
- Chobanian A, Bakris G, Black H, . National Heart, Lung, and Blood Institute; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. J Am Med Assoc. 2003;289:2560–2572.
- Mazzali M, Hughes J, Kim YG, . Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106.
- Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol. 2007;18:287–292.
- Kaplan NM, Opie LH. Controversies in hypertension. Lancet. 2006;367:168–176.
- Johnson RJ, Feig DI. Hyperuricemia in childhood essential hypertension. Hypertension. 2003;42:247–252.
- Silverstein DM, Srivaths PR, Mattison P, . Serum uric acid is associated with high blood pressure in pediatric hemodialysis patients. Pediatr Nephrol. 2011;26:1123–1128.
- Tseng CH. Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int. 2005;68:796–801.
- Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G. Hypouricemia and hyperuricemia in type 2 diabetes: Two different phenotypes. Eur J Clin Invest. 2001;31:318–321.
- Syrjänen J, Mustonen J, Pasternak A. Hypertriglyceridemia and hyperuricemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant. 2000;15:34–42.
- Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M. Serum uric acid and renal prognosis in IgA nephropathy. Nephron. 2001;87:333–339.
- Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001;24:691–697.
- Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–650.
- Domrongkitchaiporn S, Sritara P, Kitiyakara C, . Risk factors for development of decreased kidney function in a Southeast Asian population: A 12-year cohort study. J Am Soc Nephrol. 2005;16:791–799.
- Ishani A, Grandits GA, Grimm RH, . Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–1452.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–470.
- Conchol MB, Shlipak MG, Katz R, . Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50:239–247.
- Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–1211.
- Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19:2407–2413.
- Ben-Dov IZ, Kark JD. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: The Jerusalem Lipid Research Clinic cohort study. Nephrol Dial Transplant. 2011;26:2558–2566.
- Madero M, Sarnak MJ, Wang X, . Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803.
- Sturm G, Kollerits B, Neyer U, Ritz E, Kronemberg F, MMKD Study Group. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol. 2008;43:347–352.
- Mohandas R, Johnson RJ. Uric acid levels increase risk for new-onset kidney disease. J Am Soc Nephrol. 2008;19:2251–2253.
- Bradley A, Caskey CT. Hyperuricemia and urate nephropathy in urate oxidase deficient mice. Proc Natl Acad Sci USA. 1994;91:742–746.
- Nakagawa T, Mazzali M, Kang DH, . Hyperuricemia causes glomerular hypertrophy in the rat. Am J Nephrol. 2003;23:2–7.
- Watanabe S, Kang DH, Feng L, . Uric acid, hominoid evolution, and the pathogenesis salt-sensitivity. Hypertension. 2002;40:355–360.
- Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923.
- Mazzali M, Kanellis J, Han L, . Hyperuricemia induces a primary arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–F997.
- Sanchez-Lozada LG, Tapia E, Santamaria J, . Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237–247.
- Mazzali M, Kim Y-G, Suga S, . Hyperuricemia exacerbates chronic cyclosporine nephropathy. Transplantation. 2001;71:900–905.
- Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005;1:80–86.
- Nakagawa T, Hu H, Zharikov S, . A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–F631.
- Cirillo P, Sato W, Reungjui S, . Uric acid, the metabolic syndrome and renal disease. J Am Soc Nephrol. 2006;17:165–168.
- Taalat KM, El-Sheikh AR. The effect of mild hyperuricaemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27:435–440.
- Kanbay M, Ozkara A, Selcoki Y, . Effect of treatment of hyperuricaemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–1233.
- Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59.
- Afshari M, Larijani B, Rezaie A, . Ineffectiveness of allopourinol in reduction of oxidative stress in diabetic patients; A randomized, double-blind placebo-controlled clinical trial. Biomed Pharmacother. 2004;58:546–550.
- Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751.
- Kao MP, Ang DS, Gandy SJ, . Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22:1382–1389.
- Hoieggen A, Alderman MH, Kjeldsen SE, . The impact of serum uric acid in cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65(3):1041–1049.
- Miao Y, Ottenbros SA, Laverman GD, . Effect of a reduction in uric acid on renal outcomes during losartan treatment: A post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58:2–7.
- Goicoechea M, de Vinuesa SG, Verdalles U, . Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–1393.
- Halevy S, Ghislain PD, Mockenhaupt M, . Allopurinol is the most common cause of Stevens–Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008;58(1):25–32.
- Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure in adolescents with newly diagnosed essential hypertension: A randomized trial. J Am Med Assoc. 2008;300(8):924–932.
- Neal DA, Tom BD, Gimson AE, . Hyperuricemia, gout and renal function after liver transplantation. Transplantation. 2001;72:1689–1691.
- Saglam F, Celic A, Sarioglu S, . Hyperuricemia influences chronic cyclosporine nephropathy. Transplant Proc. 2008;40(1):167–170.
- Akalin E, Ganeshan SV, Winston J, Muntner P. Hyperuricemia is associated with the development of the composite outcomes of new cardiovascular events and chronic allograft nephropathy. Transplantation. 2008;86(5):652–658.
- Ejaz AA, Mu W, Kang DH, . Could uric acid have a role in acute renal failure? Clin J Am Soc Nephrol. 2007;2:16–21.
