Abstract
A case of IgA nephropathy (IgAN) associated with Crohn’s disease (CD) and preceded Helicobacter pylori (Hp) infection is described. Therapy with corticosteroids and azathioprine resulted in clinical improvement. The connection between IgAN and CD is well established, while tonsillar Hp is a potential antigen causative of IgAN. The three entities may reflect a common immunopathogenetic mechanism involving an IgA response to mucosal challenge.
INTRODUCTION
IgA nephropathy (IgAN) frequently correlates with upper respiratory and gastrointestinal (GI) tract infections, although no specific pathogen has been detected. Helicobacter pylori (Hp) is a noninvasive organism, which is the commonest causal agent of upper GI ulcers. There is an increasing volume of evidence reporting that Hp species stimulate acute and chronic inflammatory immune responses, related to extra digestive manifestations.Citation1,2 Recent studies also point out that coccoid Hp maintain outside the human body and can be identified with immunofluorescence and immunoelectron microscopy in the tonsils.Citation3
Flares and remissions of mucosal infections exaggerate systemic antibody response and are associated with the slow progression of IgAN. Additionally, the concomitant occurrence of IgAN with Crohn’s disease (CD) is well established and recent reports highlighted renal abnormalities in parallel with the activity of inflammatory bowel disease.Citation4 The failure of the intestinal inflammatory response hampers the clearance of bacteria from the tissues, provokes local chronic granulomatous inflammation, and changes the immunological status.Citation5 This article addresses a rare case of an Hp (+) patient with manifestations of IgAN and CD.
CASE REPORT
A 62-year-old Caucasian male patient with a 3-year history of complicated peptic ulcer was admitted to our hospital due to macroscopic hematuria and bloody diarrhea (3–4 stools per day). Three years ago he presented with upper GI bleeding secondary to duodenal ulcer associated with Hp infection, which showed on campylobacter-like organism (CLO)-test and antral biopsy. He successfully received a triple anti-Hp regimen (omeprazole 40 mg, clarithromycin 500 mg, amoxicillin 1 g, each administered twice daily for 10 days). The following year he had a new upper GI endoscopy with a patchy appearance of erythematous areas mainly at the antrum. Histology revealed a lymphocytic infiltrate of the gastric mucosa and confirmed the absence of Hp. Upon recent admission, he was found to be normotensive (120/80 mmHg) and afebrile with unremarkable physical examination. Upper respiratory infection episodes were not reported.
Serum laboratory data () showed hemoglobin level at 12 g/dL, hematocrit value at 37.5% with mean corpuscular volume of 90 Fl, platelet count at 369 × 103/μL, and white blood cell count at 7.1 × 103/μL with 72.1% neutrophils, 18.7% lymphocytes, 6.8% monocytes, and 0.5% eosinophils. Glucose was 107 mg/dL [normal values (NV): 70–110], serum creatinine (Cr) 2.5 mg/dL (NV: 0.6–1.0), blood urea nitrogen (BUN) 54 mg/dL (NV: 15–40), sodium 142 mEq/L (NV: 136–145), potassium 5.4 mEq/L (NV: 3.5–5.1), calcium 9.5 mg/dL (NV: 8.5–10.5), phosphorus 4 mg/dL (NV: 2.5–5), albumin 3.8 g/dL (NV: 3.4–5.0), total protein 7.4 g/dL (NV: 6.4–8.2), lactate dehydrogenase 445 U/L (NV: 100–190), uric acid 8.3 mg/dL (NV: 2.6–6.0), total cholesterol 240 mg/dL (NV: 135–200), and triglycerides 143 mg/dL (NV: 30–150). Serum liver function tests, prothrombin time, and partial thromboplastin time were normal. C-reactive protein was 2.16 mg/L (NV: <0.5), erythrocyte sedimentation rate 45, and antistreptolysin O <100. Urinalysis showed proteinuria 1+ and >100 red and 2–4 white blood cells per high power field. Urine cytology revealed red cell casts and urine culture showed no growth of bacteria. Twenty-four-hour urine measurement displayed proteinuria of 2.5 g and creatinine clearance of 50.1 mL/min/1.73 m2. Estimated glomerular filtration rate (GFR) by Modification of Diet in Renal Disease (MDRD) equation was 47.8 mL/min/1.73 m2.
Table 1. The 3-year course of laboratory tests.
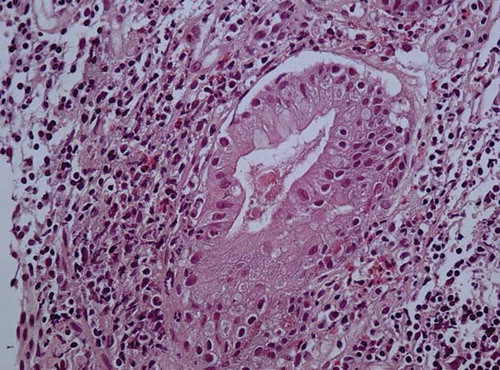
Nasogastric tube lavage and upper endoscopy excluded upper GI hemorrhage, but revealed gastritis localized at sites without histological features of Hp infection. Additionally, colonoscopy was performed disclosing small discrete aphthous ulcers adjacent to areas of normal-appearing mucosa up to 30th cm of the terminal ileum and within 20th–40th cm of the colon. Two pedunculated polyps were also found at 48 cm of colon and inflammatory changes at sigmoid. These lesions were consistent with ileocolonic CD, and intestinal biopsy specimens also reinforced the diagnosis ( and ). Abdominal ultrasonographic and chest radiographic examinations were normal. Renal ultrasound showed normal-sized kidneys with regular contours and no signs of obstruction.
Figure 1. Terminal ileum biopsies with mucosal atrophy, ulceration, and marked inflammation extending into the muscularis mucosa (H–E × 100).
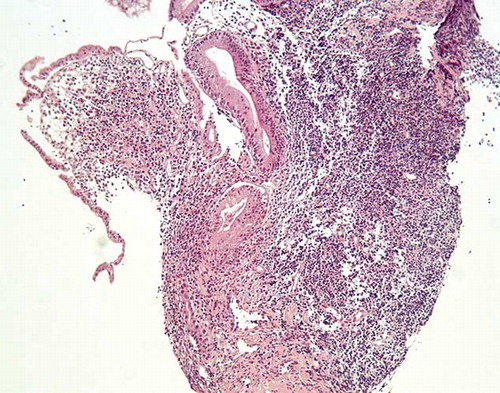
Figure 2. The lamina propia’s inflammatory cells consist of eosinophils, lymphocytes, and plasma cells with focal cryptitis (H–E × 400).
Further laboratory tests revealed serum IgA immunoglobulin of 208 mg/dL (NV: 70–410 mg/dL), IgG 813 mg/dL (NV: 690–1600 mg/dL), IgM 66 mg/dL (NV: 34–240 mg/dL), complement component 3 (C3) levels of 146 mg/dL (NV: 90–163 mg/dL), and complement component 4 levels of 44 mg/dL (NV: 16–44 mg/dL). Serum protein electrophoresis showed increased beta and gamma globulins without peak. Urine immunoelectrophoresis confirmed the presence of nonselective proteinuria and excluded any monoclonal component. Tests for antinuclear antibody, double-stranded DNA, antimitochondrial antibodies, rheumatoid factor, antineutrophilic cytoplasmic and perinuclear antibodies, anti-Sm, anti-SSA (Ro), anti-SSB (La), and anti-RNP were all negative. Serologic tests for hepatitis B and C and HIV were also negative, thyroid function was normal, and embryonic markers (alpha-fetoprotein, carcinoembryonic antigen, CA 19-9, prostatic-specific antigen) were also negative.
Due to the persistent proteinuria, hematuria, and reduction of kidney function, a left kidney percutaneous biopsy was performed. In the biopsy specimen, light microscopy revealed 23 glomeruli with 10 of them (43.4%) being completely fibrotic whereas the remainder showed extended proliferation of mesangial cells and moderately increased mesangial matrix. Small- and medium-sized vessels showed moderate intimal fibrosis, while areas of interstitial fibrosis, monocyte infiltration, and tubular atrophy were also found. Immunofluorescence examination included four glomeruli in every section with moderate (++) to intense (+++) granular staining for IgA and C3 in the mesangium and in the peripheral capillary loops. Small (+) staining for IgM was detected in the mesangium as well. The above-mentioned findings were consistent with the diagnosis of IgAN.
Therapy was initiated with oral methylprednisolone 32 mg daily and azathioprine 50 mg daily. The dose of azathioprine was not further increased, because of patient’s intolerance. As the intestinal disease improved, there was a concomitant normalization of urinary sediment, while hematuria subsided. After 2 months of therapy, protein in 24-h urine specimen was 1.5 g and renal function improved (BUN: 45 mg/dL, Cr: 1.8 mg/dL). Steroids were tapered slowly within 1 year to a maintenance dose of 4 mg/day. Renal function remained stable with serum Cr values at 1.3–1.5 mg/dL while protein 0.5 mg/24-h urine specimen and intestinal disease remained quiescent.
DISCUSSION
We described a case of IgAN diagnosed in a patient with CD and previous (3 years ago) history of GI bleeding, due to an ulcer associated with Hp infection. Whether the association of the above-mentioned entities is speculative or Hp infection influenced the occurrence and the course of the other two diseases is unknown.
The connection between IgAN and CD is well established, and Hp infection has been recognized as a causative agent of extra gastric manifestations such as acne rosacea, idiopathic chronic urticaria, and autoimmune disorders.Citation2 Although the prevalence rate of Hp infection is low in CD, these patients often have abnormalities in the upper GI tract.Citation6,7 The pathogenesis of CD and Hp infection involves immunological abnormalities and deficient or excessive expression of cytokines.Citation6
Hp species may have a pathogenic role in the development of CD and IgAN. Helicobacter hepaticus can cause severe intestinal inflammation resembling colitis and CD. Enterohepatic and gastric helicobacter species can be found in fecal specimens of children with CDCitation8 or colonizing the mucosae surface in adults as well.Citation9 Coccoid Hp was present in the palatine tonsils of patients with Hp stomach infection and with IgAN. Although coccoid Hp, sampled from the oral cavity, has not been cultivated with the current methods, reports conclude that it can be transmitted to the stomach and might be a potential IgAN-pathogenic antigen.Citation10,11
It could be speculated that Hp infection (remarked on patient’s medical history) generated a chronic mucosal immune response, which leads to mucosal IgA synthesis and circulating immune response ending in immune complex-mediated CD and IgAN. The patient continued to present gastritis, although Hp was not detected on the last antrum biopsies. Furthermore, whether the IgAN is the result of the CD remains a conundrum, because clinical symptoms of both diseases were introduced simultaneously. Usually, IgAN follows CD and the relapse of CD is associated with significant augmentation in serum IgA, which were not documented in our case.
Particularly regarding the kidney, the hypotheses share the common theme that Hp and Hp IgA antibody form immune complexes in the palatine crypts, which deposit in the glomeruli and induce IgAN.Citation3,11 This speculation is documented by the evidences below: (a) anti-Hp IgA antibodies are more frequently detected in patients with IgAN, (b) 70% of patients with IgAN revealed positive straining with anti-Hp antibody in a granular pattern along the glomerular capillary wall, (c) all patients with IgAN who had been examined for tonsillar Hp were positive, (d) IgAN patients who had undergone tonsillectomy showed better outcomes,10 and (e) elevated levels of anti-HP antibodies have also been found in patients with Henoch–Schönlein purpura, a disease characterized by IgA-containing deposits in glomerular and extraglomerular sites.Citation12
Our patient does bring up the interesting association of CD and IgAN with the history of Hp infection. The fact that serum anti-Hp antibody and tonsillar HP were not measured limited the elucidation of the pathophysiology. Systematic treatment of the intestinal disease subsided concomitantly with nephropathy, resulting in the amelioration of patient’s renal function. There have been several reported cases of IgAN associated with inflammatory bowel disease; to our knowledge, this is the first case of CD and IgAN occurring in conjunction with positive Hp infection history. Whether the connection of these diseases is a mere coincidence or they indeed share common pathogenesis deserves further investigation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Gasbarrini G, Racco S, Franceschi F, . Helicobacter pylori infection: From gastric to systemic disease. Recent Prog Med. 2010;101:27–33.
- Ohta M. Helicobacter pylori infection and autoimmune disease such as immune thrombocytopenic purpura. Kansenshogaku Zasshi. 2010;84:1–8.
- Kusano K, Inokuchi A, Fujimoto K, . Coccoid Helicobacter pylori exist in the palatine tonsils of patients with IgA nephropathy. J Gastroenterol. 2010;45:406–412.
- Filiopoulos V, Trompouki S, Hadjiyannakos D, Paraskevakou H, Kamperoglou D, Vlassopoulos D. IgA nephropathy in association with Crohn’s disease: A case report and brief review of the literature. Ren Fail. 2010;32:523–527.
- Sewell GW, Marks DJ, Segal AW. The immunopathogenesis of Crohn’s disease: A three-stage model. Curr Opin Immunol. 2009;21:506–513.
- Ando T, Nobata K, Watanabe O, . Abnormalities in the upper gastrointestinal tract in inflammatory bowel disease. Inflammopharmacology. 2007;15:101–104.
- Ando T, Watanabe O, Ishiguro K, . Relationships between Helicobacter pylori infection status, endoscopic, histopathological findings, and cytokine production in the duodenum of Crohn’s disease patients. J Gastroenterol Hepatol. 2008;23:193–197.
- Man SM, Zhang L, Day AS, Leach S, Mitchell H. Detection of enterohepatic and gastric helicobacter species in fecal specimens of children with Crohn’s disease. Helicobacter. 2008;13:234–238.
- Ge Z, Feng Y, White DA, Schauer DB, Fox JG. Genomic characterization of Helicobacter hepaticus: Ordered cosmid library and comparative sequence analysis. FEMS Microbiol Lett. 2001;204:147–153.
- Barratt J, Bailey EM, Buck KS, . Exaggerated systemic antibody response to mucosal Helicobacter pylori infection in IgA nephropathy. Am J Kidney Dis. 1999;33:1049–1057.
- Kusano K, Tokunaga O, Ando T, Inokuchi A. Helicobacter pylori in the palatine tonsils of patients with IgA nephropathy compared with those of patients with recurrent pharyngotonsillitis. Hum Pathol. 2007;38:1788–1797.
- Novák J, Szekanecz Z, Sebesi J, . Elevated levels of anti-Helicobacter pylori antibodies in Henoch-Schönlein purpura. Autoimmunity. 2003;36:307–311.
