Abstract
Aims: Several investigators have described the effect of tonsillectomy on urinary abnormalities and long-term renal survival rates in patients with IgA nephropathy (IgAN), especially during the early stage of the disease. However, whether tonsillectomy affects the rate of IgAN progress, even when the disease is in the advanced stage, remains obscure. Methods: Of 365 patients who were histologically diagnosed with IgAN, 46 eventually reached end-stage kidney disease (ESKD) between 1981 and 2006. The periods from diagnosis to ESKD with renal replacement therapy (RRT) were compared between patients with ESKD who had undergone tonsillectomy (n = 15) as initial therapy for IgAN or not (n = 31). Relationships among risk factors, initial treatment, and rates of progression to ESKD were also examined using multivariate analysis in a retrospective cohort study of the 46 patients. Results: The duration between renal biopsy and initiation of RRT was significantly extended for patients with, than without, tonsillectomy (9.8 ± 6.0 vs. 5.8 ± 4.0 years, p = 0.007; unpaired t-test). The RRT-free survival advantage in patients with tonsillectomy was also evident in Kaplan–Meier curves (p = 0.007 by log-rank test). Logistic regression analysis showed that a high serum creatinine value at biopsy and severe histological damage were risk factors affecting rapid progression (within 7 years from diagnosis) to ESKD, whereas tonsillectomy apparently delayed disease progression [odds ratio, 0.09; 95% confidence interval (CI), 0.01–0.75; p = 0.026]. Conclusion: Tonsillectomy might delay the rate of progression even when IgAN is relatively advanced, although this study could not confirm whether it prevents progression to ESKD.
INTRODUCTION
Immunoglobulin A nephropathy (IgAN) remains a common form of glomerulonephritis worldwide including Japan.Citation1–3 Nevertheless, a treatment strategy has not been established for IgAN because the mechanisms of onset and progression of the disease remain unknown.Citation4 Evidence from several credible studies has indicated that steroidsCitation5,6 and renin–angiotensin system inhibitors (RAS-I)Citation7,8 as initial therapies can improve renal outcomes, whereas the therapeutic benefit of tonsillectomy remains controversial. Although some Asian reportsCitation9–12 support the notion that tonsillectomy causes urinary abnormalities to disappear and/or renal survival rates to improve especially at the early stage of IgAN or when the disease is mild, European reportsCitation13,14 contradict these findings. This discrepancy might be associated with the backgrounds of patients differing between studies and the outcome setting (urinary findings or renal survival) against which therapeutic effects were evaluated. Under both circumstances, the ability of tonsillectomy alone to completely arrest progression to advanced IgAN seems limited.
Our previousCitation15 survey of the “point of no return” for IgAN found that reaching end-stage kidney disease (ESKD) was delayed in some patients with quite advanced IgAN at the time of diagnosis who were initially treated with tonsillectomy. Consequently, we postulated that even if tonsillectomy alone cannot completely stop advanced IgAN from progressing to ESKD, it might nevertheless delay this process. We therefore evaluated the effect of tonsillectomy on the progression of IgAN in patients who eventually reached ESKD.
METHODS
Study Design and Patients
This retrospective cohort study included 365 patients who were initially diagnosed with IgAN by renal biopsy at our institution between 1981 and 2006. Patients who had renal lesions caused by systemic diseases such as Henoch–Schönlein purpura nephritis, systemic lupus erythematosus, and liver cirrhosis were excluded. During an observation period of 77.6 ± 56.0 (5–273) months, 46 (12.6%) of these patients reached ESKD. We investigated the clinical syndromes at initial visit, laboratory findings at diagnosis, and initial therapy for the patients.
Evaluation of Clinical Syndromes at Initial Visit and Parameter at Diagnosis
Clinical syndromes at the initial presentation at our hospital were defined as asymptomatic hematuria and/or proteinuria, macroscopic hematuria, acute, chronic, and rapidly progressive nephritic syndrome, and nephrotic syndrome according to the modified classification of World Health OrganizationCitation16 by the Research Group on progressive Renal Disease in Japan.Citation17
Methods of measuring serum creatinine (sCr) at our institution changed in 1986 from the Jaffé to an enzymatic procedure. Therefore, the sCr values measured by the Jaffé method between 1981 and 1986 were calibrated to the values generated by the enzymatic method by subtracting 0.207 mg/dL, based on data from Japanese patients reported by Imai et al.Citation18 Hypertension was defined as systolic blood pressure (BP) > 140 mmHg and/or diastolic BP > 90 mmHg or the use of antihypertensive medication before diagnosis. Creatinine clearance was calculated from 24-h urine samples.
The end point of this study was ESKD requiring renal replacement therapy (RRT) such as hemodialysis, peritoneal dialysis, and renal transplant.
Assessment of Histological Severity
We assessed histological lesions from all patients with IgAN according to the guidelines of the Special Society Group (IgAN) on Progressive Glomerular Disease in Japan.Citation19 These guidelines separate patients with IgAN based on severity into Grades 1–4 as follows:
Grade 1: Slight mesangial cell proliferation and increased matrix; absence of glomerulosclerosis, crescent formation, or adhesion to Bowman’s capsule; no prominent changes in interstitium, renal tubuli, or blood vessels.
Grade 2: Slight mesangial cell proliferation and increased matrix; glomerulosclerosis, crescent formation, or adhesion to Bowman’s capsule in <10% of all biopsied glomeruli; interstitial and vascular findings identical to those of Grade 1.
Grade 3: Moderate, diffuse mesangial cell proliferation and increased glomerulosclerosis, crescent formation, or adhesion to Bowman’s capsule in 10–30% of all biopsied glomeruli; slight cellular infiltration in interstitium, except around some sclerosed glomeruli, slight tubular atrophy, and mild vascular sclerosis.
Grade 4: Severe, diffuse mesangial cell proliferation and increased matrix; glomerulosclerosis, crescent formation, or adhesion to Bowman’s capsule in >30% of all biopsied glomeruli. When sites of sclerosis are totaled and converted to global sclerosis, the sclerosis rate includes >50% of glomeruli; some glomeruli also show compensatory hypertrophy, interstitial cellular infiltration, tubular atrophy, and fibrosis; hyperplasia or degeneration evident in some intrarenal arteriolar walls.
Statistical Analysis
All continuous variables are presented as means ± standard deviation (SD). Clinical parameters of the two groups were compared using the unpaired t-test for normally distributed continuous variables or the Mann–Whitney U-test for non-normally distributed continuous variables. Differences in proportions were evaluated using the χ2 independent test or the Fisher exact test for 2 × 2 tables, depending on the number of categories. Histological grades between two groups were compared using Pearson’s χ2-test. The RRT-free survival rates of the two groups were analyzed using the Kaplan–Meier method, and differences in survival curves were compared using the log-rank test. We used the logistic regression model to assess the impact of multiple covariates on the rate of progression to ESKD. All of the independent variables used in multivariate analyses are expressed as categorical (coded as 0/1) or quantitative forms. Initial treatment (tonsillectomy and steroid therapy) was regarded as a categorical variable. Age, systolic BP, urinary protein value, sCr value, and histological grade were regarded as quantitative variables. The results of the multivariate analysis are expressed as odds ratios with a 95% confidence interval (CI). A p-value of <0.05 was considered statistically significant in all analyses, which were performed by SPSS for Windows, Advance Statistical Release 11.0 (SPSS, Chicago, IL, USA).
RESULTS
Characteristics of Patients Who Reached ESKD
shows a comparison of clinical syndromes at initial presentation between patients with and without ESKD at the final observation. The ratios (%) of chronic nephritic syndrome and nephrotic syndrome were significantly higher in patients with, than without, ESKD (chronic nephritic syndrome, 41.3% vs. 16.6%; nephrotic syndrome, 28.3% vs. 6.3%, p < 0.001). The baseline data of 46 patients with ESKD at diagnosis shown in revealed high BP (136.0 ± 20.5 mmHg), massive proteinuria (2.77 ± 1.77 g/day), elevated sCr (1.30 ± 0.85 mg/dL), and severe histological damage (Grade 4 in 57% of all patients). Among the patients, 15 (32.6%) had undergone tonsillectomy as the initial therapy of IgAN. The following numbers of patients were diagnosed during the following periods: 8 (17.4%), 14 (30.4%), 16 (34.9%), 6 (13.0%), and 2 (4.3%) between 1981 and 1985, 1986 and 1990, 1991 and 1995, 1996 and 2000, and 2001 and 2005, respectively. Therefore, reflecting the historical background, steroid therapy and RAS-I including angiotensin-converting enzyme inhibitors (ACE-I) and/or angiotensin receptor blockers (ARB) were administered only to seven (15.2%) and five (10.9%) patients, respectively. Although the clinical and pathological data at diagnosis did not differ between patients treated or not by tonsillectomy, the duration between diagnosis and ESKD was significantly longer for those who had undergone tonsillectomy (9.8 ± 6.0 vs. 5.8 ± 4.0 years; p = 0.007 by unpaired t-test; ).
Table 1. Comparison of clinical syndromes at initial presentation.
Table 2. Baseline characteristics of patients.
Comparison of RRT-Free Time with and without Tonsillectomy
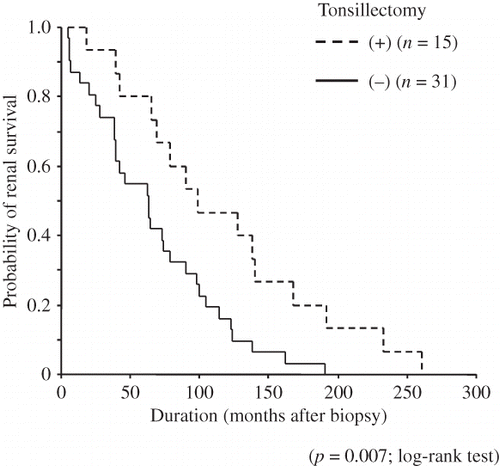
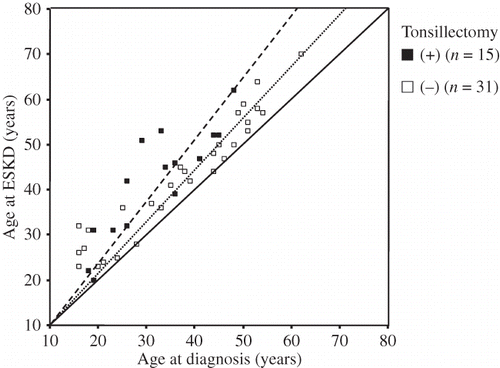
shows the relationship between age at diagnosis of IgAN and at ESKD requiring RRT for all patients. The average of the ratio of age at ESKD and at diagnosis was higher in patients with, than without, tonsillectomy (age at ESKD = 1.29 vs. 1.13 × age at diagnosis). shows that the RRT-free survival advantage in patients with tonsillectomy was also evident in Kaplan–Meier curves (p = 0.007, log-rank test). The 10-year renal survival rates of patients with and without tonsillectomy were 46.7% and 16.1%, respectively.
Figure 1. Relationship between age at diagnosis and at ESKD in patients.
Notes: Patients with (▪) and without (□) tonsillectomy. Slopes of lines show ratio of age at ESKD to that at diagnosis (with tonsillectomy, large broken line; age at diagnosis = 1.29 × age at diagnosis; patients without tonsillectomy, small broken line; age at ESKD = 1.13 × age at diagnosis).
Multivariate Analysis of Factors Affecting Rate of Progression to ESKD
Since the mean period from diagnosis to ESKD was 7.0 ± 5.1 years for all patients, we set the cut off for multivariate analysis using the logistic regression model at 7 years to evaluate factors affecting progression to ESKD within the various periods. Tonsillectomy, steroid therapy, and risk factors for the progression to ESKD such as BP, urinary protein, sCr, and histological severity were selected as imperative independent variables in the model. RAS-I was excluded from the model because only five patients received this medication. The results showed that a high sCr value at diagnosis and severe histological damage were risk factors affecting rapid progression (within 7 years of diagnosis) to ESKD, whereas tonsillectomy apparently delayed disease progression with an odds ratio of 0.09 (95% CI, 0.01–0.75, p = 0.026; ).
Table 3. Logistic regression analysis of factors affecting ESKD progression within 7 years in 46 patients with IgAN.
DISCUSSION
Although the influence of mucosal immunity (particularly that of the tonsils) on IgAN has remained obscure, it has been considered since the 1980s as a pathogenetic mechanism of the onset and progression of IgAN.Citation20,21 Some recent basic studies have provided important information and suggestions to resolve this issue. Aberrant IgA1 glycosylation might be a key pathogenetic factor contributing to the formation of immune complexes and their deposition in the mesangium in IgAN.4,22–24 Itoh et al.Citation25 found using an antisynthetic IgA1 hinge peptide antibody that some aberrantly glycosylated IgA1 is derived from the tonsillar mucosa. Suzuki et al.Citation26 re-evaluated the concept of a disordered “mucosa-bone marrow axis”Citation27,28 using their established IgAN-prone mouse model. Their findings indicated that the tonsils comprise a major mucosal site of new priming and a reservoir for pathogenic IgA-producing cells and that tonsillectomy reduces the subsequent dissemination of these cells into other tissues such as bone marrow.
However, the contradictory results of several studies have rendered the value of tonsillectomy alone as a treatment for IgAN debatable. Xie et al.Citation9 indicated a significant advantage of tonsillectomy for long-time renal survival compared with no treatment (89.6% vs. 63.7% at 20 years by Kaplan–Meier analysis). Akagi et al.Citation10 found significantly improved urinary factors (disappearance of proteinuria and hematuria) and renal survival rates in patients with, than without, tonsillectomy. Our previous study also found that urinary abnormalities disappear at a significantly higher frequency in patients treated with tonsillectomy and that tonsillectomy independently contributes to renal survival in multivariate analysis.Citation11 These studies from Japan have some analogies for the backgrounds of the patients; over half of treated patients have normal renal function and mild proteinuria (<1.0 g/day), that is, the disease is at an early stage or it is a mild form. Although a study from ChinaCitation12 could not prove a statistical advantage of tonsillectomy for long-term renal survival (mean follow-up, 11 years) in a treated group with almost the same severity of disease (mean Cr, 1.08 mg/dL; mean proteinuria, 0.98 g/day), the Kaplan–Meier curves were similar to those of Xie (p = 0.059, log-rank test) in the report described above.Citation9 On the other hand, Rasche et al.Citation13 found that tonsillectomy did not impact the renal survival of patients with advanced IgAN (mean sCr in treated group, 2.36 mg/dL) with a mean follow-up of 3.4 years. A recent long-time follow-up (20 years) study from ItalyCitation14 did not find an effect of tonsillectomy on the progression of mesangioproliferative glomerulonephritis in a patient cohort that included 61 (34% of the total) patients with mild IgAN. However, that study did not distinguish between IgAN and non-IgAN in the multivariate analysis, and the outcome setting was progression to stage 3 CKD. Although these conflicting results might be associated with different levels of disease severity at initial presentation and different outcome settings from the viewpoint of study design, these results can be summarized as the ability of tonsillectomy to improve urinary abnormalities and long-term renal survival in patients with mild IgAN and normal renal function and the inability to improve renal survival in those with advanced IgAN and impaired renal function.
This study used a lateral approach to examine the effect of tonsillectomy on renal prognosis and analyzed “RRT-free time” from diagnosis to ESKD. Therefore, we selected study patients with advanced IgAN at diagnosis who eventually reached ESKD. We found that the RRT-free time was extended in patients who had undergone tonsillectomy compared with those who had not, and multivariate analysis confirmed the contribution of tonsillectomy to this delay in the rate of IgAN progression even when the disease was quite advanced. These findings imply that tonsillectomy can retard, but not prevent, disease progression in patients with advanced IgAN and encourage implementing another treatment strategy such as steroid therapy and RAS-I. In fact, our previous cohort studyCitation29 verified that a patient group with tonsillectomy and a high frequency of steroid and RAS-I use had a significantly favorable prognosis, compared with those who had undergone tonsillectomy alone. A recent meta-analysisCitation30 also found that tonsillectomy combined with either oral steroid or steroid pulse therapy induced higher rates of clinical remission (disappearance of hematuria and proteinuria) with favorable long-term effects, whereas tonsillectomy alone did not significantly increase remission rates over those obtained with general treatment. These clinical results support the notion that tonsillectomy alone cannot directly suppress extant inflammation in glomerular cells, although it might prevent new attacks against glomerular cells. Therefore, a combination of steroid and/or RAS-I therapy should be considered for patients with IgAN except when the disease is in the early stage.
This study has several limitations that should be considered. First, the study was a retrospective analysis of a small sample of patients. Second, we studied data from 38 of the 46 patients who were diagnosed between 16 and 30 years ago. This was because we wished to investigate only patients who had required RRT. Third, multivariate analysis found that steroid administration did not contribute to favorable renal survival. This might have been influenced by the small number of patients who received steroid therapy. RAS-I was excluded from the multivariate model because it had been administered to even fewer patients. The lower frequency of steroid and RAS-I administration to the study patients was linked to the period of diagnosis. Therefore, we could not evaluate the effect of either steroid or RAS-I alone in this study. Finally, some race-related factors might have relation with the favorable result of tonsillectomy in this study because all of the study patients were Japanese.
The present findings suggest that tonsillectomy can delay the rate of progression even when IgAN is quite advanced, although we could not determine whether it prevents progression to ESKD. Nevertheless, our findings implied that the value of tonsillectomy alone as a curative treatment for advanced IgAN is limited. Several reportsCitation31–33 refer to an effect of tonsillectomy on patients receiving oral immunosuppressive therapy for recurrent IgAN after kidney transplantation. Moreover, tonsillectomy combined with steroid pulse therapy induces a high ratio of clinical remission at the relatively early stage of IgAN, and this should become a curative treatment modality.Citation34,35 Future clinical and experimental studies are needed to determine the pathogenesis of IgAN including mucosal immunity.
ACKNOWLEDGMENTS
This study was presented in part at the 41st Annual Meeting of the American Society of Nephrology, 4–9 November 2008, in Philadelphia, PA, USA.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709–727.
- Julian BA, Waldo FB, Rifai A, Mestecky J. IgA nephropathy, the most common glomerulonephritis worldwide: A neglected disease in the United States? Am J Med. 1988;84:129–132.
- Sugiyama H, Yokoyama H, Sato H, . Committee for Standardization of Renal Pathological Diagnosis and Working Group for Renal Biopsy Database, Japanese Society of Nephrology, Tokyo, Japan. Japan Renal Biopsy Registry: The first nationwide, web-based, and prospective registry system of renal biopsies in Japan. Clin Exp Nephrol. 2011;15:493–503.
- Floege J. The pathogenesis of IgA nephropathy: What is new and how does it change therapeutic approaches? Am J Kidney Dis. 2011; 58:992–1004.
- Cheng J, Zhang X, Zhang W, He Q, Tao X, Chen J. Efficacy and safety of glucocorticoids therapy for IgA nephropathy: A meta-analysis of randomized controlled trials. Am J Nephrol. 2009;30:315–322.
- Zhou YH, Tang LG, Guo SL, . Steroids in the treatment of IgA nephropathy to the improvement of renal survival: A systematic review and meta-analysis. PLoS One. 2011;6:e18788.
- Praga M, Gutierrez E, Gonzalez E, Morales E, Hernandez E. Treatment of IgA nephropathy with ACE inhibitors: A randomized and controlled trial. J Am Soc Nephrol. 2003;14:1578–1583.
- Coppo R, Peruzzi L, Amore A, . IgACE: A placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol. 2007;18:1880–1888.
- Xie Y, Nishi S, Ueno M, . The efficacy of tonsillectomy on long-term renal survival in patients with IgA nephropathy. Kidney Int. 2003;63:1861–1867.
- Akagi H, Kosaka M, Hattori K, . Long-term results of tonsillectomy as a treatment for IgA nephropathy. Acta Otolaryngol Suppl. 2004;Suppl. 555:38–42.
- Komatsu H, Fujimoto S, Hara S, . Multivariate analysis of prognostic factors and effect of treatment in patients with IgA nephropathy. Ren Fail. 2005;27:45–52.
- Chen Y, Tang Z, Wang Q, . Long-term efficacy of tonsillectomy in Chinese patients with IgA nephropathy. Am J Nephrol. 2007;27:170–175.
- Rasche FM, Schwarz A, Keller F. Tonsillectomy does not prevent a progressive course in IgA nephropathy. Clin Nephrol. 1999;51:147–152.
- Piccoli A, Codognotto M, Tabbi MG, Favaro E, Rossi B. Influence of tonsillectomy on the progression of mesangioproliferative glomerulonephritis. Nephrol Dial Transplant. 2010;25:2583–2589.
- Komatsu H, Fujimoto S, Sato Y, . “Point of no return (PNR)” in progressive IgA nephropathy: Significance of blood pressure and proteinuria management up to PNR. J Nephrol. 2005;18:690–695.
- Churg J, Bernstein J, Glassock RJ. Renal Disease: Classification and Atlas of Glomerular Disease. 2nd ed., Tokyo: Igaku-Shoin; 1995:4–20.
- Koyama A, Igarashi M, Kobayashi M, . Natural history and risk factors for immunoglobulin A nephropathy in Japan. Am J Kidney Dis. 1997;29:526–532.
- Imai E, Horio M, Nitta K, . Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11:41–50.
- Tomino Y, Sakai H. Clinical guidelines for immunoglobulin A (IgA) nephropathy in Japan, second version. Clin Exp Nephrol. 2003;7:93–97.
- Bene MC, Hurault De Ligny B, Kessler M, Faure GC. Confirmation of tonsillar anomalies in IgA nephropathy: A multicenter study. Nephron. 1991;58:425–428.
- Bene MC, Hurault Ligny B, Kessler M, Foliguet B, Faure GC. Tonsils in IgA nephropathy. Contrib Nephrol. 1993;104:153–161.
- Barratt J, Smith AC, Feehally J. The pathogenic role of IgA1 O-linked glycosylation in the pathogenesis of IgA nephropathy. Nephrology (Carlton). 2007;12:275–284.
- Tanaka M, Seki G, Someya T, Nagata M, Fujita T. Aberrantly glycosylated IgA1 as a factor in the pathogenesis of IgA nephropathy. Clin Dev Immunol. 2011;2011:470803.
- Novak J, Moldoveanu Z, Julian BA, . Aberrant glycosylation of IgA1 and anti-glycan antibodies in IgA nephropathy: Role of mucosal immune system. Adv Otorhinolaryngol. 2011;72:60–63.
- Itoh A, Iwase H, Takatani T, . Tonsillar IgA1 as a possible source of hypoglycosylated IgA1 in the serum of IgA nephropathy patients. Nephrol Dial Transplant. 2003;18:1108–1114.
- Suzuki Y, Tomino Y. Potential immunopathogenic role of the mucosa-bone marrow axis in IgA nephropathy: Insights from animal models. Semin Nephrol. 2008;28:66–77.
- van den Wall Bake AW, Daha MR, Evers-Schouten J, van Es LA. Serum IgA and the production of IgA by peripheral blood and bone marrow lymphocytes in patients with primary IgA nephropathy: Evidence for the bone marrow as the source of mesangial IgA. Am J Kidney Dis. 1988;12:410–414.
- van den Wall Bake AW, Daha MR, Radl J, . The bone marrow as production site of the IgA deposited in the kidneys of patients with IgA nephropathy. Clin Exp Immunol. 1988;72:321–325.
- Komatsu H, Fujimoto S, Hara S, . Recent therapeutic strategies improve renal outcome in patients with IgA nephropathy. Am J Nephrol. 2009;30:19–25.
- Wang Y, Chen J, Wang Y, Chen Y, Wang L, Lv Y. A meta-analysis of the clinical remission rate and long-term efficacy of tonsillectomy in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:1923–1931.
- Kennoki T, Ishida H, Yamaguchi Y, Tanabe K. Proteinuria-reducing effects of tonsillectomy alone in IgA nephropathy recurring after kidney transplantation. Transplantation. 2009;88:935–941.
- Ushigome H, Suzuki T, Fujiki M, . Efficacy of tonsillectomy for patients with recurrence of IgA nephropathy after kidney transplantation. Clin Transplant. 2009;23(Suppl. 20):17–22.
- Tsuchiya T, Ito S, Yamaguchi Y, Moriyama Y, Ehara H, Deguchi T. Tonsillectomy and steroid pulse therapy for recurrent IgA nephropathy in renal allograft. Clin Nephrol. 2010;73:68–71.
- Hotta O, Miyazaki M, Furuta T, . Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis. 2001;38:736–743.
- Komatsu H, Fujimoto S, Hara S, Sato Y, Yamada K, Kitamura K. Effect of tonsillectomy plus steroid pulse therapy on clinical remission of IgA nephropathy: A controlled study. Clin J Am Soc Nephrol. 2008;3:1301–1307.