Abstract
Aim: Apoptosis plays a critical role in the pathogenesis of gentamicin (Gen)-induced nephrotoxicity. However, the underlying molecular mechanisms still remain unclear. In this study, we addressed the role of p38 mitogen-activated protein kinase (MAPK)/inducible nitric oxide synthase (iNOS) signaling pathway in Gen-induced nephrotoxicity and evaluated the protective effect of the free-radical scavenger N-acetylcysteine amide (NACA). Methods: Pig kidney epithelial cells (LLC-PK1) cells were exposed to Gen for variable times and doses. Cytotoxicity was assessed by morphology and by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Protein expression was assessed by Western blotting. Results: Exposure to Gen-induced apoptosis in a dose-dependent and time-dependent manner was assessed by DNA content analysis and poly ADP ribose polymerase (PARP) cleavage. Gen caused increased phosphorylation of p38 MAPK and induction of iNOS. This was accompanied by a significant upregulation of Bax and nuclear factor κB (NF-κB) and a downregulation of Bcl-2 expression. Pretreatment with SB203580, aminoguanidine (AG), and NACA inhibited apoptosis. Furthermore, pretreatment with SB203580 and NACA not only attenuated the pro-apoptotic effect of Gen, but also significantly reversed its effects on p38 MAPK phosphorylation and iNOS induction. The Gen-induced effects on Bcl-2, Bax, and NF-κB expression were also reversed by SB203580, AG, and NACA. Conclusion: In conclusion, NACA can attenuate Gen-induced apoptotic injury in LLC-PK1 cells through inhibiting p38 MAPK/iNOS signaling pathway.
INTRODUCTION
The use of the aminoglycoside antibiotic gentamicin (Gen) in the treatment of Gram-negative bacterial infections can be significantly limited by its nephrotoxicity, which can cause acute renal failure even at the lowest therapeutic doses. One identified cytotoxic mechanism of Gen is the induction of apoptosis in proximal renal tubular cells and mesangial cells,Citation1–3 which can be reproduced in cultured renal cells at high extracellular concentrations (1–3 mM).Citation4 Although these findings suggest that apoptosis may be an important mechanism in Gen-induced nephrotoxicity, the underlying molecular mechanisms still remain unclear.Citation5
Oxidative stress in the form of accumulation of reactive oxygen species has been suggested to mediate Gen-induced apoptosis through activation of cysteine-dependent aspartate-directed protease, caspase.Citation6,7 We have recently demonstrated an apoptotic cascade mediated by p38 mitogen-activated protein kinase (MAPK) and inducible nitric oxide synthase (iNOS) in iohexol-induced apoptosis in renal proximal tubular epithelial cells and that this cascade can be blocked by the thiol-containing free-radical scavenger N-acetylcysteine amide (NACA or AD4).
However, little is known about p38 MAPK/iNOS signaling pathway in Gen-induced apoptosis. In this study, we investigate the role of p38 MAPK/iNOS signaling pathway in Gen-induced apoptosis and evaluate the protective effect of NACA on Gen-induced apoptosis in pig kidney epithelial cells (LLC-PK1) cells.
MATERIALS AND METHODS
Reagents
All chemicals were purchased from Sigma (St. Louis, MO, USA) unless indicated otherwise. Cell culture medium and plastic ware were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). NACA was provided by Dr. Glenn Goldstein (David Pharmaceuticals, New York, NY, USA).
Cell Culture and Treatments
LLC-PK1 cell line from porcine proximal tubule (European Collection of Animal Cell Cultures, Porton Down, Wiltshire, UK) was cultured in Dubelcco’s modified Eagle’s medium supplemented with 10% fetal calf serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) in an atmosphere of 5% CO2 in air at 37°C. Experiments were performed on cells of passages 5–12. The cells were plated at a density of 5 × 104 cells per cm2 and only confluent cell monolayers with “dome formation” were studied.
The selective iNOS inhibitor aminoguanidine [AG, 10 mM, freshly dissolved in phosphate buffered solution (PBS)] and p38 MAPK inhibitor SB203580 [5 μM, freshly dissolved in dimethyl sulfoxide (DMSO)] were added 30 min before cells were exposed to 3 mM Gen. NACA (5 mM, freshly dissolved in culturing media) was added to the cell culture at the same time of adding Gen.
MTT Assay of Cell Viability
Cell viability was assessed and quantified using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) cytotoxicity assay as described previously.Citation8 Cells were harvested in log-phase growth using trypsin:EDTA, then seeded on 96-well plates at a density of 1 × 104 cells per well, and cultured at 37°C for 20 h. In brief, cells were exposed to different concentrations of Gen at 37°C. After treatment with Gen, 15 μL of dye solution was added to each well, and the plates were incubated for 4 h at 37°C. Then, 100 μL of solubilization solution/stop mix was added to each well, and the plates were left overnight in a sealed container with a humidified atmosphere to completely solubilize the formazan crystals. The absorbance was measured at 570 nm against 650 nm as the reference.
MTT conversion rate (MTT%) was calculated as follows:
Morphology
Morphological changes were routinely checked under phase contrast microscope. To compare the concentration dependency and time dependency of Gen-induced cell injury, a broad range of Gen concentration (1–12 mM) and exposure time (12–72 h) were investigated.
Analyses of Cell Apoptosis (PI Staining) by Fluorescence-Activated Cell Sorter (FACS)
Apoptotic cells show diminished DNA content below the G0/G1 population of normal diploid cells.Citation9 In order to count the cells in sub-diploid, flow cytometry was used by the method of cell staining with propidium iodide (PI). Briefly, cell pellets were fixed in 70% ethanol at –20°C for at least 24 h. After being washed twice with ice-cold PBS, cells were incubated in RNase A/PBS (1 mg/mL) at 37°C for 30 min. DNA was stained with PI for 15 min at room temperature and analyzed by flow cytometry on a linear scale using a Becton Dickinson fluorescence-activated cell sorter (FACS) Caliber apparatus (Becton Dickinson and Co., San Jose, CA, USA).
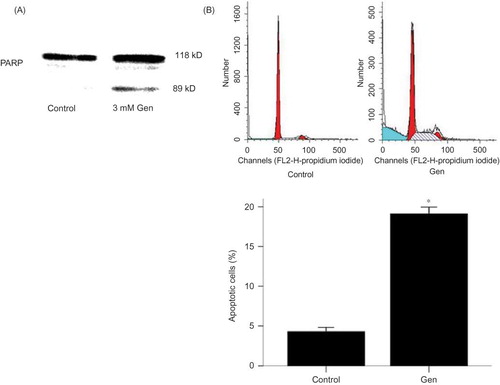
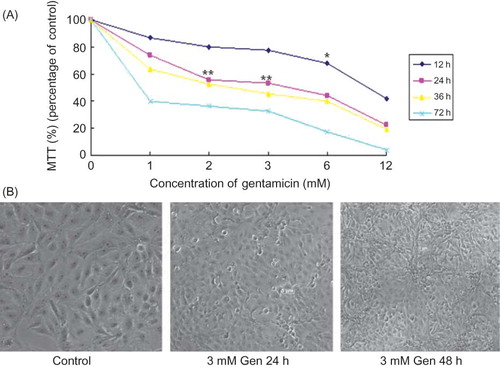
Figure 1. Exposure to Gen induces apoptosis in a time-dependent and dose-dependent manner. LLC-PK1 cells were incubated with Gen at different concentrations for 12, 24, 36, or 72 h. Cell viability was determined by the MTT assay (A), and morphology was examined by phase contrast microscopy at ×100 magnification (B). The values are mean ± SD (n = 3).
Notes: *p < 0.05 versus control. **p < 0.01 versus control.
Cells within a distinct sub-G0/G1 peak were considered apoptotic, due to DNA condensation and fragmentation, which resulted in reduced PI staining. Approximately 1 × 104 counts were performed for each sample. Apoptosis rate was calculated by evaluating the percentage of events accumulated in the pre-G0/G1 position with ModFit LT for Mac 3.0 software (Becton Dickinson and Company). Experiments were performed in triplicates and repeated 3 times.
Protein Extraction and Western Blotting Analysis
Western blotting analysis was performed as described.Citation8 In brief, cells were lysed for 15 min in lysis buffer [62.5 mmol/L Tris–HCl (pH 6.8), 6 mol/L urea, 10% glycerol, 2% sodium dodecyl sulfate, and 5% β-mercaptoethanol] and then sonicated 3 times for 3 s each. The protein concentration of the extracts was estimated with the Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules, CA, USA) using bovine serum albumin (BSA) as standard. Total proteins (30 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 6–12% polyacrylamide gel. The proteins in the gel were transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% skim milk in Tris-buffered saline with Tween-20 [0.05% v/v Tween-20 in tris-buffered saline (TBS), pH 7.2] for 1 h. Membranes were incubated with primary antibody at 4°C overnight, followed by the addition of horseradish peroxidase-labeled secondary antibody for 1 h. The primary antibodies were diluted in tris-buffered saline Tween-20 (TBST) with 5% w/v nonfat dry milk or 5% w/v BSA. Membranes were washed 3 times in TBST for 10 min between each step. Antibody labeling was detected using an enhanced chemiluminescence reagent (NEN™ Life Science, Boston, MA, USA) according to the manufacturer’s instructions. Protein expression was quantified by ImageJ 1.45 software (Wayne Rasband, NIH, Bethesda, MD, USA) after scanning the film.
The specific primary antibodies included the following: anti-iNOS (Sigma, 1:5000); anti-poly ADP ribose polymerase (anti-PARP) (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:1000); anti-phospho-p38 MAPK (Santa Cruz Biotechnology, 1:1000); anti-Bcl-2 (Santa Cruz Biotechnology, 1:1000); anti-Bax (Santa Cruz Biotechnology, 1:1000); anti-nuclear factor κB (NF-κB) (Santa Cruz Biotechnology, 1:1000); and anti-β-actin (Santa Cruz Biotechnology, 1:1000). All the experiments were performed at least 3 times under the same conditions.
Statistical Analysis
All data are presented as mean ± SD. Statistical significance was assessed with one-way analysis of variance (ANOVA) (SPSS statistical analysis software, version 15.0, SPSS Inc., Chicago, IL, USA). In some cases, a t-test was performed between two groups. Differences were considered significant if p < 0.05 and highly significant if p < 0.01.
RESULTS
Gen Induces Apoptosis in Cultured LLC-PK1 Cells
LLC-PK1 cells exhibited a time-dependent and dose-dependent reduction in viability after 12–72 h of exposure to 1, 2, 3, 6, and 12 mM Gen as analyzed by a MTT assay (A). Microscopically, this was accompanied by cell shrinkage, membrane blebbing, and brightly rounded bodies under phase contrast microscopy, indicating the occurrence of apoptosis (B). In subsequent experiments, cells were exposed to 3 mM Gen for 24 h.
Induction of apoptosis was also indicated by cleavage of caspase substrate PARP by Western blotting, a biochemical hallmark of apoptosis (A). To quantify the apoptosis, the sub-G0/G1 DNA contents of PI-stained fixed cells were analyzed by FACS. The percentage of apoptotic cells was increased from 4.29 ± 0.44% to 19.10 ± 0.69% (p < 0.01 vs. control) (B).
Gen-Induced Apoptosis Is Mediated by iNOS
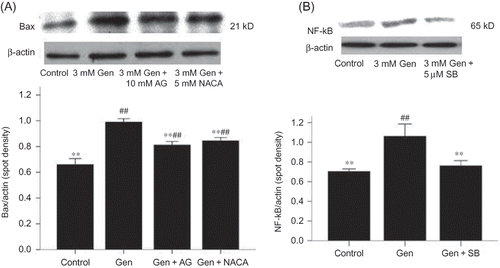
Western blotting revealed an induction of iNOS expression following exposure to Gen (A). The selective iNOS inhibitor AG was used to explore the potential role of iNOS activation in Gen-induced cell apoptosis. This demonstrated that AG significantly reduced Gen-induced apoptosis as assayed by morphological changes (A), PARP cleavage (B), and FACS analysis (C). In addition, the rise in iNOS protein following Gen exposure was completely blocked by the addition of AG (A).
Figure 3. Exposure to Gen induces activations of iNOS, p38 MAPK, NF-κB, and Bax, as well as inhibition of Bcl-2. Alteration in iNOS (A), phospho-p38 MAPK (B), NF-κB (C), Bcl-2 (D), and Bax (E) after exposure to Gen for 24 h was detected by Western blotting. The values are mean ± SD (n = 3).
Note: *p < 0.01 versus Gen treatment.
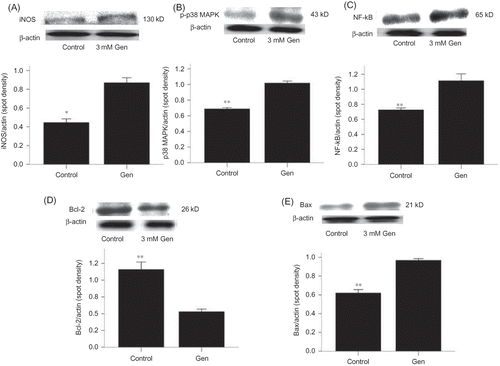
Figure 4. Protection against Gen-induced apoptosis by iNOS inhibitor AG, p38 inhibitor SB203580, and NACA. (A) Phase contrast microscopy at ×100 magnification. (B) PARP cleavage by Western blotting. (C) Apoptosis rate by FACS. The values are mean ± SD (n = 5).
Notes: **p < 0.01 versus Gen treatment. #p < 0.05 versus control. ##p < 0.01 versus control.
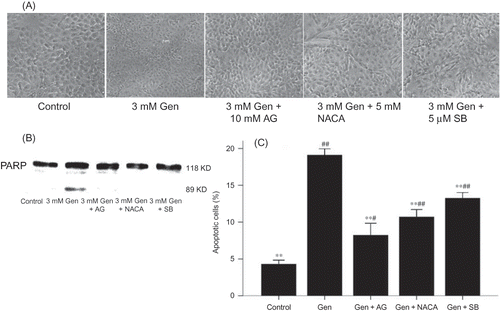
Figure 5. Effects of AG, SB203580, and NACA on iNOS (A). Protein expression and effects of NACA and SB203580 on phosphor-p38 MAPK protein expression (B). The values are mean ± SD (n = 3).
Notes: *p < 0.05 versus Gen treatment. **p < 0.01 versus Gen treatment. #p < 0.05 versus control. ##p < 0.01 versus control.
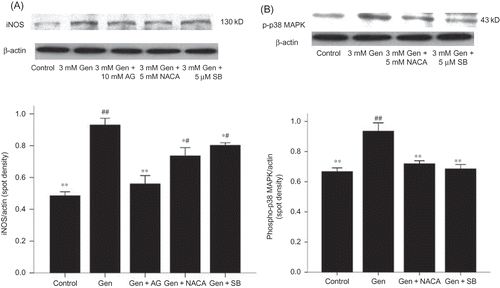
Gen Induces iNOS by Activation of p38 MAPK
We examined the activation of p38 MAPK by Western blotting using phospho-specific antibody. Western blotting showed an activation of p38 MAPK following exposure to Gen (B).
To further investigate this, a specific p38 MAPK inhibitor (SB203580) was used. Inhibition of p38 MAPK attenuated apoptosis as assayed by morphological changes (), PARP cleavage (B), and FACS (C). Furthermore, SB203580 also attenuated the Gen-induced increase in iNOS protein expression, indicating that p38 MAPK activation is upstream of the iNOS activation in the signaling cascade induced by Gen (A).
Gen-Induced Apoptosis Is Associated with Activation of the Redox-Sensitive Transcription Factor NF-κB and Altered Expression of the Anti-apoptotic Protein Bcl-2 and the Pro-apoptotic Protein Bax
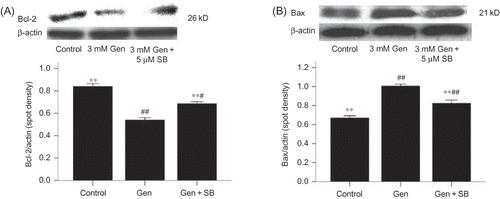
An activation of NF-κB following exposure to Gen was demonstrated by increased nuclear levels of NF-κB p65 (C). This activation could be inhibited by blocking the signal at the p38 MAPK activation (B) or iNOS activation (A).
Figure 6. Effects of AG and NACA on NF-κB and Bcl-2 protein expression. The values are mean ± SD (n = 3).
Notes: **p < 0.01 versus Gen treatment. #p < 0.05 versus control. ##p < 0.01 versus control.
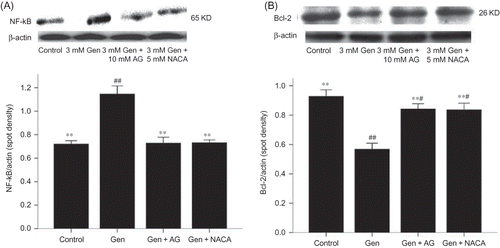
Gen exposure also decreased the expression of the anti-apoptotic protein Bcl-2 (D) and increased the expression of the pro-apoptotic protein Bax (E). Again, these effects could be blocked at the p38 MAPK activation (A and B) or iNOS activation (B and A).
NACA Protects against Gen-Induced Apoptosis
Pretreatment with 0.5–5 mM NACA resulted in a dose-dependent inhibition in Gen-induced apoptosis with maximal inhibition at 5 mM as assayed by morphological changes (A) and PARP cleavage (B). In all subsequent experiments 5 mM NACA was used. The protective effect of NACA was further evidenced by the significant decrease in apoptotic DNA fragmentation (C).
Western blotting revealed that NACA inhibited the p38 MAPK activation triggered by Gen (B), as well as the downstream events in the signal pathway: iNOS induction (A), NF-κB activation (A), and alteration in the expression of Bax and Bcl-2 (B and A).
DISCUSSION
In this study, we investigated the molecular mechanisms of Gen-induced nephrotoxicity in cultured LLC-PK1 cells. The characteristic apoptotic morphological features were elicited by a significantly higher Gen concentration than the target plasma concentration aimed for in antibiotic therapy.Citation10,11 This phenomenon has been reported previouslyCitation3,12,13 and may reflect low uptake of Gen in vitro.Citation4,13
p38 MAPK plays an important role in inflammatory responses and apoptosis.Citation14 Here we report that Gen causes p38 MAPK phosphorylation and iNOS induction and that the specific p38 MAPK inhibitor SB203580 and the selective iNOS inhibitor AG reduce Gen-induced apoptosis. This confirms a previous report that p38 MAPK is involved in Gen-induced apoptosis.Citation15 Since iNOS induction by Gen was inhibited by the p38-MAPK inhibitor SB203580, we suggest that p38 MAPK acts upstream of iNOS in the cascade of Gen-induced apoptosis. Induction of iNOS by p38 MAPK has been demonstrated previously,Citation16–18 and iNOS-derived NO is a key molecule in tubular epithelial apoptosis induced by different toxic agents.Citation19–21 One mechanism by which NO can induce apoptosis is through increased expression or translocation of the pro-apoptotic protein Bax from cytosol to mitochondria.Citation22,23 In agreement with this, we observed Gen-induced increased expression of the pro-apoptotic protein Bax and decreased expression of the anti-apoptotic protein Bcl-2.
A number of signal paths have been identified downstream of p38 MAPK activation in regulating diverse cellular functions.Citation18,24,25 This study demonstrates that Gen induces a p38 MAPK-mediated signal leading to the activation of the NF-κB and that the p38 MAPK inhibitor SB203580 could block this.
We have previously reported that the antioxidant NACA can block contrast-mediated nephrotoxicity.Citation8 Therefore, we investigated whether NACA could inhibit Gen-induced apoptosis. Our results demonstrate that NACA blocks Gen-induced apoptosis by suppression of p38 MAPK activation and iNOS protein expression and by reversing the effects of Gen on Bcl-2, Bax, and NF-κB expression. However, we found that neither NACA nor SB203580 could completely block Gen-induced apoptosis. This suggests the presence of additional pathways contributing to Gen-induced apoptosis.
In summary, we have demonstrated an apoptotic cascade triggered by p38 MAPK-mediated iNOS and NF-κB in renal tubular proximal epithelial cells exposed to Gen. Moreover, the antioxidant NACA prevents Gen-induced apoptosis by blocking this signal pathway. NACA may thus prevent Gen-induced nephropathy and other complications associated with the induction of p38 MAPK and iNOS.
ACKNOWLEDGMENT
We thank Dr. Glenn Goldstein for providing N-acetylcysteine amide.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- El Mouedden M, Laurent G, Mingeot-Leclercq MP, Tulkens PM. Gentamicin-induced apoptosis in renal cell lines and embryonic rat fibroblasts. Toxicol Sci. 2000;56:229–239.
- Martinez-Salgado C, Eleno N, Morales AI, Perez-Barriocanal F, Arevalo M, Lopez-Novoa JM. Gentamicin treatment induces simultaneous mesangial proliferation and apoptosis in rats. Kidney Int. 2004;65:2161–2171.
- Yang TH, Young YH, Liu SH. EGb 761 (Ginkgo biloba) protects cochlear hair cells against ototoxicity induced by gentamicin via reducing reactive oxygen species and nitric oxide-related apoptosis. J Nutr Biochem. 2011;22(9):886–894.
- Servais H, Jossin Y, Van Bambeke F, Tulkens PM, Mingeot-Leclercq MP. Gentamicin causes apoptosis at low concentrations in renal LLC-PK1 cells subjected to electroporation. Antimicrob Agents Chemother. 2006;50:1213–1221.
- Juan SH, Chen CH, Hsu YH, . Tetramethylpyrazine protects rat renal tubular cell apoptosis induced by gentamicin. Nephrol Dial Transplant. 2007;22:732–739.
- Cuzzocrea S, Mazzon E, Dugo L, . A role for superoxide in gentamicin-mediated nephropathy in rats. Eur J Pharmacol. 2002;450:67–76.
- Reiter RJ, Tan DX, Sainz RM, Mayo JC, Lopez-Burillo S. Melatonin: Reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol. 2002;54:1299–1321.
- Gong X, Celsi G, Carlsson K, Norgren S, Chen M. N-acetylcysteine amide protects renal proximal tubular epithelial cells against iohexol-induced apoptosis by blocking p38 MAPK and iNOS signaling. Am J Nephrol. 2010;31:178–188.
- Polat A, Parlakpinar H, Tasdemir S, . Protective role of aminoguanidine on gentamicin-induced acute renal failure in rats. Acta Histochem. 2006;108:365–371.
- Denamur S, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Apoptosis induced by aminoglycosides in LLC-PK1 cells: Comparative study of neomycin, gentamicin, amikacin, and isepamicin using electroporation. Antimicrob Agents Chemother. 2008;52:2236–2238.
- Servais H, Van Der Smissen P, Thirion G, . Gentamicin-induced apoptosis in LLC-PK1 cells: Involvement of lysosomes and mitochondria. Toxicol Appl Pharmacol. 2005;206:321–333.
- Santucci RA, Krieger JN. Gentamicin for the practicing urologist: Review of efficacy, single daily dosing and “switch” therapy. J Urol. 2000;163:1076–1084.
- Gibbons CE, Maldonado-Perez D, Shah AN, Riccardi D, Ward DT. Calcium-sensing receptor antagonism or lithium treatment ameliorates aminoglycoside-induced cell death in renal epithelial cells. Biochim Biophys Acta. 2008;1782:188–195.
- Kwon CH, Yoon CS, Kim YK. Ciglitazone induces caspase-independent apoptosis via p38-dependent AIF nuclear translocation in renal epithelial cells. Toxicology. 2008;244:13–24.
- Volpini RA, Balbi AP, Costa RS, Coimbra TM. Increased expression of p38 mitogen-activated protein kinase is related to the acute renal lesions induced by gentamicin. Braz J Med Biol Res. 2006;39:817–823.
- Ozbek E, Cekmen M, Ilbey YO, Simsek A, Polat EC, Somay A. Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF-kappaB pathways. Ren Fail. 2009;31:382–392.
- Rupesh KR, Priya AM, Sundarakrishnan B, Venkatesan R, Lakshmi BS, Jayachandran S. 2,2′-Bipyridyl based copper complexes down regulate expression of pro-inflammatory cytokines and suppress MAPKs in mitogen induced peripheral blood mononuclear cells. Eur J Med Chem. 2010;45:2141–2146.
- Kole L, Giri B, Manna SK, Pal B, Ghosh S. Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFkappaB nuclear translocation. Eur J Pharmacol. 2011;653:8–15.
- Liu H, Bargouti M, Zughaier S, . Osteoinductive LIM mineralization protein-1 suppresses activation of NF-kappaB and selectively regulates MAPK pathways in pre-osteoclasts. Bone. 2010;46:1328–1335.
- Serbecic N, Beutelspacher SC. Anti-oxidative vitamins prevent lipid-peroxidation and apoptosis in corneal endothelial cells. Cell Tissue Res. 2005;320:465–475.
- Jang SH, Lim JW, Kim H. Beta-carotene inhibits Helicobacter pylori-induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in human gastric epithelial AGS cells. J Physiol Pharmacol. 2009;60(Suppl. 7):131–137.
- Al-Majed AA, Mostafa AM, Al-Rikabi AC, Al-Shabanah OA. Protective effects of oral arabic gum administration on gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 2002;46:445–451.
- Ghatan S, Larner S, Kinoshita Y, p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol. 2000;150:335–347.
- Vause CV, Durham PL. CGRP stimulation of iNOS and NO release from trigeminal ganglion glial cells involves mitogen-activated protein kinase pathways. J Neurochem. 2009;110:811–821.
- Kar S, Ukil A, Sharma G, Das PK. MAPK-directed phosphatases preferentially regulate pro- and anti-inflammatory cytokines in experimental visceral leishmaniasis: Involvement of distinct protein kinase C isoforms. J Leukoc Biol. 2010;88:9–20.