Abstract
Calcific uremic arteriolopathy (CUA) is a rare but life-threatening disorder of arteriolar calcification. It frequently leads to severe ischemia, intense pain, and tissue necrosis with non-healing skin ulcerations. CUA usually occurs in patients with chronic kidney disease (CKD), especially those on dialysis, and its occurrence is rare in kidney transplant recipients. The treatment of this disorder is not clearly defined, and no randomized prospective trials are available. Treatment has focused on optimizing dialysis treatment, control of bone mineral parameters, wound care, experimental anticalcification therapies—using bisphosphonates, cinacalcet, parathyroidectomy, and hyperbaric oxygen. Such treatments are based on the pathophysiological considerations and evidences from case reports or series. Recently, several cases have reported about the emerging benefits of intravenous sodium thiosulfate (STS) in the treatment of CUA. STS has resulted in rapid pain relief, wound healing, and prevention of death. We report a case of CUA in a 63-year-old Caucasian man with a functioning renal allograft. In this patient, intravenous STS was administered for 8 months, which was the principal therapy, which resulted in complete resolution of the CUA and skin healing.
INTRODUCTION
Calcific uremic arteriolopathy (CUA), also known as calciphylaxis, is a serious complication of renal failure. It is characterized by vascular and other soft tissue calcification, intimal hypertrophy, and thrombosis of small vessels that results in painful tissue necrosis, which often leads to ulceration and secondary infection. CUA predominantly occurs in patients with advanced chronic kidney disease (CKD) on renal replacement therapy, and this can include renal transplant recipients. The known prevalence is approximately 1–4% of patients on hemodialysis.Citation1 Twenty-one cases of CUA were reported in renal transplant recipients.Citation2 CUA has also been reported in non-uremic patients with primary hyperparathyroidism and with autoimmune diseases.Citation3
CUA is associated with a high mortality rate of between 60% and 80% due to superimposed infection leading to overwhelming sepsis even with maximal therapies.Citation4,5 The reported therapeutic options are few in number, and the outcomes are poor. The use of sodium thiosulfate (STS) to treat CUA in dialysis-dependent patients has been described in the literature. We report here a case of CUA in a renal transplant recipient, who was successfully treated with STS.
CASE REPORT
A 63-year-old Caucasian man with end-stage kidney failure secondary to IgA nephropathy received his first deceased donor renal transplantation in 2000. This en bloc allograft was eventually lost to a chronic rejection in 2003, and he returned to maintenance hemodialysis. Aortic valve incompetence and an aortic arch aneurysm secondary to Marfan’s syndrome required an aortic valve (mechanical) and aortic root replacement in 2006 and an ongoing requirement for warfarin. He received a second deceased donor renal transplant in 2008. The postoperative course was uncomplicated despite thepresence of a donor-specific antibody, and his immunosuppressive treatment included tacrolimus, mycophenolate mofetil, and prednisolone.
In December 2009, he presented with a 4-week history of painful, non-healing posttraumatic ulcer on his right medial calf (A). Distal arterial pulses were present. An ultrasound examination of his lower limbs did not reveal deep vein thrombosis. Laboratory investigations revealed the following: impaired allograft function with a serum creatinine of 170 μmol/L (reference range, RR: 50–120 μmol/L) and eGFR 34 mL/min/1.73 m2. Serum calcium, phosphate, and calcium–phosphate product were 2.37 mmol/L (RR: 2.1–2.5 mmol/L), 0.94 mmol/L (RR: 0.65–1.45 mmol/L), and 2.23, respectively. He had hyperparathyroidism with a parathyroid hormone (PTH) level of 84.6 pmol/L (RR: 0.8–5.5 pmol/L). A skin biopsy demonstrated extensive calcification and endovascular fibrosis of small subcutaneous arteries that were consistent with CUA ().
Figure 1. (A) Early stage of calcific uremic arteriolopathy (CUA) skin lesions on the right medial calf. (B) Progressive CUA skin lesions appear as deep necrotic ulcers on the bilateral lower limbs. (C) Resolution of CUA skin lesions after STS treatment.
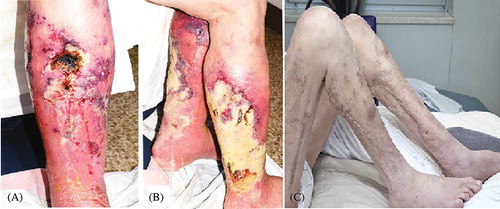
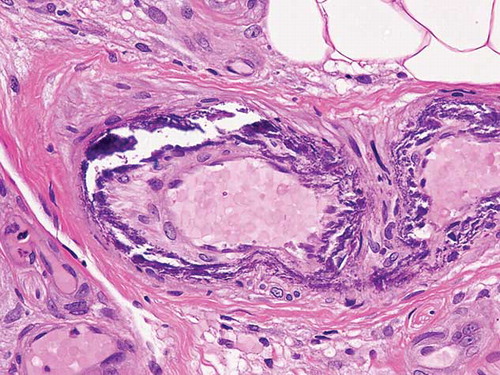
Figure 2. Histology demonstrating the extensive medial calcification of the small-sized arteries in the subcutaneous tissue, which is typical of calcific uremic arteriolopathy (CUA).
Treatment initially comprised broad-spectrum antibiotics and aggressive wound care. Parathyroidectomy was not performed as anticoagulation was an ongoing requirement. Instead, he was treated with cinacalcet 30 mg daily. He also received two sessions of hyperbaric oxygen therapy, which was then stopped because the target oxygen level in the tissue was not reached. Warfarin was ceased and replaced with intravenous sodium heparin infusion. Despite these aggressive treatments and biochemical improvement in PTH level, the wound progressively worsened and involved both lower limbs (B). STS (25 g intravenously in 40 mL of normal saline over 60 min) was administered three times a week and was commenced 10 weeks after initial presentation. Within a week, there was a dramatic reduction in pain and the skin lesions did not progress. He then underwent wound debridement on several occasions with split-skin grafting. STS was well tolerated. This was continued for a total of 4 months and then changed to once a week for another 4 months. In the 6 months following STS withdrawal, the patient was pain-free and the wounds were completely healed without recurrence (C). Intravenous sodium heparin was switched to intravenous enoxaparin and then subcutaneous enoxaparin, with treatment being monitored by the antifactor Xa levels 4 h post dose. Renal allograft function remained stable throughout the course of treatment with STS ().
Table 1. Serum creatinine, calcium–phosphate parameters, and acid–base status before, during, and after sodium thiosulfate therapy.
DISCUSSION
CUA is being reported with increased frequency in the post-transplant settings. There are now more than 21 cases reported of CUA in renal transplant recipients in the English medical literature.Citation2 The use of STS in CUA has been reported in more than 14 hemodialysis and peritoneal patients, and 1 patient with stage 2 CKD.Citation6,7 STS has also been trialed in patients with normal renal function.Citation8 However, the case reported here is the first to use STS for the treatment of CUA in an immunocompromised renal transplant recipient.
Many risk factors have been proposed for CUA with hyperparathyroidism, hyperphosphatasemia, elevated calcium–phosphate product of >70 mg2/dL2, obesity (BMI > 30 kg/m2), liver disease, systemic corticosteroid usage, warfarin therapy, and a serum albumin concentration less than 25 g/L, being found to place individuals at risk of developing CUA.Citation4
The pathogenesis of CUA is not fully understood. Disturbances of calcium–phosphate homeostasis are considered the main pathogenic event leading to CUA. Calcium hydroxyapatite is observed to be deposited in small- and medium-sized blood vessels followed by intimal proliferation, endovascular fibrosis, and intravascular thrombosis. Obliterative vasculopathy then leads to tissue ischemia, necrosis, and poor healing. Endothelial dysfunction due to oxidative stress and reactive oxygen species has also been hypothesized to play a role in the pathogenesis of CUA.Citation9 The occurrence of CUA in transplant recipients is further complicated by concomitant immunosuppression. It has been suggested that acute rejection and therapy with pulse steroids is an important risk factor for CUA with high mortality.Citation2
Treatment of CUA involves multiple modalities including control of hyperphosphatasemia using non-calcium containing phosphate binders, reduction of calcium–phosphate product, correction of hyperparathyroidism surgically (subtotal parathyroidectomy) or medically (cinacalcet), removal of warfarin, aggressive wound care, and treatment of infection. Unfortunately, none of these modalities has shown consistent beneficial results, and the prognosis for patients with CUA remains very poor. Other treatment modalities include the use of bisphosphonates, hyperbaric oxygen, and STS.Citation5,10,11
STS, a reducing agent that forms water-soluble complexes with many metals, is approved for use in cyanide poisoning as a chelator of cations, for the topical treatment of acne, and as a chemoprotectant against auditory neuronal toxicity associated with carboplatin or cisplatin chemotherapy.Citation12 The first successful use of STS in the treatment of CUA was reported in 2004,Citation11 and since then many cases have reported on the use of STS in the treatment of CUA.Citation6 Only one patient treated with STS had previously received a kidney transplant, but this patient was returned to hemodialysis at the time of developing CUA.Citation13
The mechanism of action of STS in the treatment of CUA is not well understood. It is thought that STS chelates calcium and thus ameliorates precipitation in the skin, subcutaneous tissues, and various organs.Citation14 Recently, Farah et al.Citation15 demonstrated the deposition of iron in affected microvasculature, thus suggesting a potential role for iron in the pathogenesis of CUA, and this provides another rationale for the use of STS. In addition, STS has antioxidant activity, restoring endothelial production of nitric oxide and promoting vasodilatation.Citation14
STS is absorbed poorly from the gastrointestinal tract. After intravenous administration, 28.5% of STS is cleared unchanged by glomerular filtration while the remainder is metabolized in the liver to sulfate.Citation16 The half-life has been estimated to be 182 min following a 150 mg/kg intravenous infusion. In patients on hemodialysis, the serum half-life is prolonged to 478 min.Citation17 Pharmacokinetic data in patients with mild renal impairment, transplant recipients, and those on peritoneal dialysis are unknown. The usual dose is 25 g intravenously in 40 mL normal saline over 60 min three times a week. Brucculeri et al. reported response to only 5 g intravenous STS four times a week. The duration of treatment varies, but it is suggested that STS should be continued for at least 3 months after the complete healing of skin ulceration.Citation11,18
Of the reported CUA patients treated with STS, where there was a positive response to STS, pain relief was reported within several days but dermal lesions took weeks or months to heal. Where reported, the duration of therapy with STS ranged between 6 weeks and 34 months.
Caution is recommended with the use of STS in patients with renal impairment because thiocyanate is eliminated renally; however, no patient has developed thiocyanate intoxication. To date, there are no reports of worsening renal function in non-dialysis dependent CUA patients treated with STS.Citation7,8 Other adverse reactions to STS include hypotension—which is dose-related, rhinorrhea, sinus congestion, nausea and vomiting, headache, and hallucination. Our case did not experience any of these adverse reactions. Two previous cases reported adverse reaction in the form of nausea and vomiting, which lead to discontinuation of STS in one case and dose reduction in another.Citation19 STS has also been associated with increased anion gap metabolic acidosis because of the unmeasured accumulation of thiosulfuric acid.Citation17,18,20 In addition, the combination cinacalcet and STS can result in severe hypocalcaemia.Citation18
No study has yet compared the efficacy of various CUA treatment modalities in a prospective controlled manner. The low numbers of patients in individual centers would make a randomized controlled trial of treatment in CUA difficult if not impossible. Although the literature, limited to case reports, supports the use of STS in patients with CUA, further prospective study is needed to fully determine the potential benefits of STS in the treatment of CUA.
CUA is a life-threatening condition and its pathophysiology remains to be fully elucidated. The optimal management of CUA is unknown at present because of its infrequent occurrence and the lack of prospective randomized studies. STS has emerged as a well-tolerated and effective treatment in the dialysis population, and this case report demonstrates, for the first time, its effectiveness and safety in a renal transplant recipient.
Declaration of interest: We have had no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated. This manuscript has not been published or submitted for publication elsewhere.
REFERENCES
- Angelis M, Wong LL, Myers SA, Wong LM. Calciphylaxis in patients on hemodialysis: A prevalence study. Surgery. 1997;122:1083–1090.
- Aabed G, Furayh OA, Al-Lehbi A, Al Mana H, Al Ghamedi A, Helmy A. Calciphylaxis-associated second renal graft failure and patient loss: A case report and review of literature. Exp Clin Transplant. 2008;4:287–293.
- Nigwekar SU. Calciphylaxis from nonuremic causes: A systematic review. Clin J Am Soc Nephrol. 2008;3:1139–1143.
- Weenig RH, Sewell LD, Davis MD, . Calciphylaxis: Natural history, risk factor analysis and outcome. J Am Acad Dermatol. 2007;56:569–579.
- Rogers NM, Chang SH, Teubner DJO, Coates PTH. Hyperbaric oxygen as effective adjuvant therapy in the treatment of distal calcific uremic arteriolopathy. Nephrol Dial Transplant Plus. 2008;4:244–249.
- Colette BR, Wazny LD. Sodium thiosulfate, bisphosphonates, and cinacalcet for treatment of calciphylaxis. Am J Health-Syst Pharm. 2008;65:1419–1429.
- Hackett BC, McAleer MA. Calciphylaxis in a patient with normal renal function, response to treatment with sodium thiosulfate. Clin Exp Dermatol. 2009;34:39–42.
- Baker BL, Fitzgibbons CA, Buescher LS. Calciphylaxis responding to sodium thiosulfate therapy. Arch Dermatol. 2007;123:269–270.
- Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: The emerging role of sodium thiosulfate. Cardiovasc Diabetol. 2005;4:4.
- Monney P, Nguyen QV, Perroud H, Descombes E. Rapid improvement of calciphylaxis after intravenous pamidronate therapy in a patient with chronic renal failure. Nephrol Dial Tranplant. 2004;19:2130–2132.
- Cicone JS, Petronis JB, Embert CD, Spector DA. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104–1108.
- Sodium thiosulfate. In: Klasco RK, ed. DRUGDEX. Greenwood Village, CO: Thomson Micromedex; 2007.
- Ackermann F, Levy A, Daugas E, . Sodium thiosulfate as first-line treatment for calciphylaxis. Arch Dermatol. 2007;143:1336–1337.
- Hayden MR, Kolb LG, Khanna R. Calciphylaxis and the cardiometabolic syndrome. J Cardiometab Syndr. 2006;1:76–79.
- Farah M, Crawford RI, Levin A, Chan YC. Calciphylaxis in the current era: Emerging “Ironic” features? Nephrol Dial Transplant. 2011;26:191–195.
- Braverman B, Ivankovich AD, Shah G. Thiosulfate pharmacokinetics in normal and anuric dogs. Proc Soc Exp Biol Med. 1982;170:273–280.
- Brucculeri M, Cheigh J, Bauer G, Serur D. Long-term intravenous sodium thiosulphate in the treatment of a patient with calciphylaxis. Semin Dial. 2005;18:431–434.
- Araya CE, Fernnell RS, Neiberger RE, Dharnidharka VR. Sodium thiosulfate treatment for calcific uremic arteriolopathy in children and young adults. Clin J Am Soc Nephrol. 2006;1:1161–1166.
- Tokashiki K, Ishida A, Kouchi M, . Successful management of critical limb ischemia with intravenous sodium thiosulphate in a chronic hemodialysis patient. Clin Nephrol. 2006;66:140–143.
- Mataic D, Bastani B. Intraperitoneal sodium thiosulphate for the treatment of calciphylaxis. Ren Fail. 2006;28:361–363.
