Abstract
Background/objectives: Cardiovascular disease begins early in the course of chronic kidney disease (CKD), and the glomerular filtration rate (GFR) is an independent risk factor for it. There is little information on cardiac troponin concentrations in patients with CKD who have not commenced dialysis. Factors associated with this deleterious process are not completely understood, and we aimed to determine associated laboratory abnormalities of increased cardiac troponin T (cTnT) in patients with CKD. Methods: In this study, 104 patients (65 males and 39 females with mean age of 65 ± 15 years) were recruited. A detailed clinical history was recorded and routine biochemical variables and cTnT levels were measured. GFR was estimated (44.62 ± 14.38 mL/min/1.73 m2) using the modification of diet in renal disease study formula. Results: cTnT is correlated with blood urea (r = 0.262, p < 0.05), uric acid (r = 0.399, p < 0.001), blood phosphorus (r = 0.550, p < 0.001), triglyceride (r = 0.329, p = 0.011), C-reactive protein (CRP; r = 0.768, p < 0.001), renal resistive index (RRI; r = 0.412, p = 0.017), and GFR (r = −0.755, p = 0.011). On stepwise multiple regression analysis, increased CRP (≥12 mg/L), uric acid (≥5 mg/L), and RRI (≥0.70) were independent variables for increased cTnT status (r2 = 0.053, p < 0.05). Conclusion: Increased cTnT not only shows ongoing inflammation but also is a sensitive marker of functioning renal mass. It is strongly correlated with factors influencing the decline in renal function; thus, it can be used as a renal risk parameter.
INTRODUCTION
Cardiovascular disease is prevalent and the leading cause of morbidity and mortality in patients with end-stage renal disease (ESRD).Citation1 Cardiovascular risk begins early in the course of ESRD, and the glomerular filtration rate (GFR) is an independent risk factor for cardiac events.Citation2,3
Cardiac troponin T (cTnT) is a low molecular weight protein and an integral component of the myofibrillar contractile apparatus of the heart. However, high rates of false positivity have been reported in patients with renal failure.Citation4–7 Using cutoff ≥0.01 μg/L, the prevalence of cTnT elevation is reported to range from 30% to 85% in patients with ESRD.Citation8,9 Although the exact mechanism is not known, it has been proposed that subclinical myocyte damage and apoptosis due to uremia or decreased clearance due to renal failure may be the underlying cause for the higher levels of cardiac markers in this group of patients.Citation10,11 In fact, the debate about the interpretation of elevated cTnT in the presence of abnormal renal function is still ongoing.
Evidence suggests that increased cTnT concentrations in uremic patients do indeed reflect myocardial injury. However, increases in serum cardiac troponin concentration can occur in the absence of ischemic events, including among patients with renal failure.Citation12,13 There is little information on cardiac troponin concentrations in patients with ESRD who have not commenced dialysis. However, factors associated with this deleterious process are not completely understood, and there is robust evidence that cTnT is a powerful prognostic marker in the ESRD. We studied pre-dialysis patients with moderate and severe kidney failure to address the following questions: (a) At which stage does the progression of kidney disease cause an increase in cTnT concentration? (b) Is there a relationship between renal failure parameters and cTnT concentrations in pre-dialysis patients?
METHODS
Patients
This study is a cross-sectional analysis of cTnT levels in patients with different stages of chronic kidney disease (CKD). A group of 104 patients with CKD attending our nephrology outpatient clinic were recruited to the study. Inclusion criteria were patients had stage 3 or stage 4 CKD, were ≥18 years of age, and who were able to understand and sign the consent form. Patients with angiographically proven stenosis (>50% of the luminal diameter), acute coronary syndromes, history of myocardial infarction, chronic stable angina pectoris, previous coronary revascularization, regional wall-motion abnormalities in echocardiography, significant ECG changes suggestive of myocardial ischemia, acute renal failure, functioning renal transplant or receiving dialysis, and recent (<6 months) cardiac event were excluded from the study. A detailed clinical history was recorded, the study was approved by the local ethical committee, and written informed consent was obtained from all patients prior to study entry. The study was conducted according to the declaration of Helsinki.
Measurement of Biochemical Parameters
Blood and urine samples were collected in the fasting state. The serum and EDTA plasma aliquots were stored at −70°C until analysis. Serum cTnT tests were assayed using ELISA test kits (DRG Instruments GmbH, Marburg, Germany), and other biochemical variables were measured by standardized methods using auto analyzers. GFR was estimated (44.62 ± 14.38 mL/min/1.73 m2) using the modification of diet in renal disease study formula. Patients were stratified into stage 3 CKD (moderate; eGFR = 30–59 mL/min/1.73 m2) and stage 4 CKD (severe; eGFR = 15–29 mL/min/1.73 m2) according to guidelines.Citation14
Ultrasonographic and Echocardiographic Examinations
Doppler examinations were all performed by the same operator using Sonoline Elegra Cx 5-2 multi-D array transducer (Siemens Medical Solutions USA, Malvern, PA, USA). Patients were placed in a supine position, and measurements were performed on segmental and interlobar arteries to give the best Doppler signal both for the quantity of flow and for the correct angle. Waveforms with a clearly represented early systolic peak were used for the determination of the renal resistive index (RRI). To obtain the best possible definition of the early systolic peak, the sample gate was reduced to the size of the vascular lumen, and the pulse repetition frequency was continuously adapted to the arterial blood flow velocity at the point where the gate was positioned. The RRI was calculated as follows: RRI = (peak systolic velocity − end diastolic velocity)/peak systolic velocity. The threshold for an increased RRI was at least 0.70 because this value has been shown to be a discriminatory RRI level.Citation15
Patients underwent two-dimensional targeted M-mode echocardiography using Hewlett Packard, Sonos 7500 (Andover, MA, USA). Left ventricular mass (LVM) was calculated using the formula of Devereux and indexed to body surface area to obtain the LVM index (LVMI). Left ventricular hypertrophy (LVH) was considered present when LVMI ≥ 125 g/m2.Citation16,17
Data Analysis
Descriptive statistics for all the identified variables (age, sex, and dialysis age) were performed. The results were given as mean ± SD (normally distributed data). The differences between the three groups were assessed either by parametric (one-way ANOVA and Turkey’s test) or by nonparametric (χ2-test) test. For the comparison of the groups, Mann–Whitney U-test was used for nonparametric variables and χ2-test for parametric variables. p-Values less than 0.05 were considered statistically significant. The SPSS version 11.0 (SPSS, Inc., Chicago, IL, USA) was used to perform all statistical calculations.
RESULTS
Patients’ characteristics for the whole study population and by CKD stage are given in . The mean age was 58 ± 15 years; 65 patients (63%) were male, and 43 patients (41%) had stage 3 CKD. Overall, serum cTnT concentrations were increased (≥0.01 μg/L) in 56 of 104 (54%) patients. Serum cTnT differed significantly between CKD stages, being more commonly increased in the presence of more advanced CKD [increased in 18 of 43 (41%) and 38 of 61 (62%) patients with stage 3 and stage 4 CKD, respectively; p = 0.01].
Table 1. Patient characteristics by CKD stage.
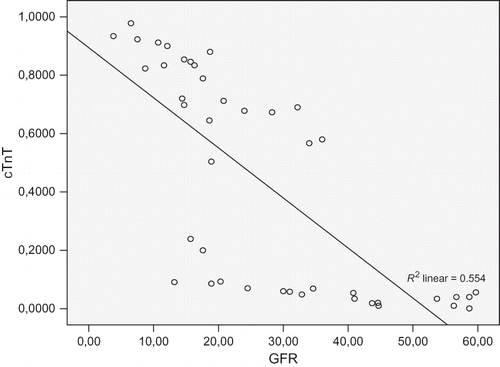
Serum cTnT has positive correlation with blood urea (r = 0.262), uric acid (r = 0.399), blood phosphorus (r = 0.550), triglyceride (r = 0.329), C-reactive protein (CRP; r = 0.768), RRI (r = 0.412), LVMI (r = 0.173), and negative correlation with hemoglobin (r = −0.028) and GFR (r = −0.755, all p < 0.05).
There were significant associations between increased cTnT (≥0.01 μg/L) and increased uric acid, total cholesterol, serum CRP, leukocyte, RRI, and LVMI (p = 0.04, p = 0.02, p = 0.00, p = 0.03, p = 0.01, and p = 0.01, respectively). Increased cTnT levels were significantly associated with decreased hemoglobin levels (p = 0.01, ). Increased cTnT levels were associated with a diminished eGFR (OR = 0.96; 95% CI, 0.93–0.99; p = 0.01; ).
Table 2. Parameters related with increased cTnT levels.
Echocardiographic data were available for all patients: the prevalence of LVH was 46% in all patients. There were correlations between increased LVMI with increased systolic blood pressure (r = 0.34, p = 0.01) and age (r = 0.39, p = 0.03) and decreased eGFR (r = −0.61; p = 0.01). The prevalence of increased cTnT (≥0.01 μg/L) was higher in patients with LVH (56% vs. 32%, p < 0.01).
On stepwise multiple regression analysis, increased CRP (≥12 mg/L), uric acid (≥5 mg/L), and RRI (≥0.70) were independent variables for increased cTnT status (r2 = 0.053, p < 0.05).
DISCUSSION
Renal insufficiency alters the metabolism of many proteins; thus, the clinical significance of elevated cardiac biomarkers in patients with ESRD is hard to interpret. For CKD patients who are not receiving dialysis, data are limited and conflicting.Citation18–20 To our knowledge, this is the first study to document the relatively high prevalence of increased cTnT with the degree of renal failure in pre-dialyzed patients. In this study, we demonstrated that there are significant elevations of troponin levels with renal function parameters in CKD patients.
In our study, using the cutoff of ≥0.01 μg/L, the prevalence of cTnT elevation is 54% in patients with CKD. Goicoechea et al. noted cTnT concentrations (≥0.01 μg/L) in a lower proportion (16%) of ESRD patients than in this study.Citation21 The increased prevalence of increased cTnT concentrations in our study compared with the study of Goicoechea et al. probably reflects the relatively inferior renal function in our study (59% of our patients had stage 4 CKD and in their study very few patients had kidney failure). And relating to the data in our study, serum cTnT differed significantly between CKD stages, being more commonly increased in the presence of more advanced CKD (increased cTnT incidence is 41% and 62% in patients with stage 3 and stage 4 CKD, respectively; p = 0.01).
In our study, we found that CRP levels were significantly and independently correlated with cTnT levels. Zoccali et al. reported a similar independent association between CRP and cTnT in ESRD patients.Citation22 This is a finding that draws attention to the link between inflammation and atherosclerosis, and CRP is now being viewed as a nontraditional risk factor for ESRD patients.Citation23 Increased cTnT levels have been found in association with pro-inflammatory cytokines such as CRP or IL-6 in the literature, and studies reported a positive correlation between troponin levels and CRP in these patient groups.Citation23,24 cTnT may be a useful and more sensitive marker than hs-CRP in predicting atherosclerotic risk in ESRD.
In this study, we also found that uric acid levels were significantly correlated with cTnT levels. The associations between uric acid and cardiac markers in CKD patients have not been studied in the literature. Elevated serum uric acid level is a risk factor for cardiovascular disease and our findings may suggest that uric acid levels may serve as a predictive marker for atherosclerosis in ESRD patients as well. Uric acid has deleterious effects on endothelial function, platelet adhesion and aggregation, and nitric oxide bioavailability.Citation25 Uric acid may mediate the hypertension by dysfunction of endothelial and vascular smooth muscle cells resulting in oxidative stress, reduction in endothelial nitric oxide, and activation of the renin–angiotensin system.Citation26
There was a significant negative correlation with eGFR and positive correlation with RRI between elevated serum cTnT concentrations. The pathophysiologic mechanisms causing increased cTnT concentrations in patients with kidney disease are not clear. The increased frequency of RRI in patients with higher cTnT levels might be explained in part by kidney damage, and decreasing clearance from the kidney may contribute to the elevated cTnT in patients with CKD. RRI reflects renal microvascular disease. Since GFR correlates with renal plasma flow, low eGFR in our study might be a result of renal microvascular disease. In advanced CKD (diabetes in particular), macrovascular renal disease occurs simultaneously.
Improvement in renal function after renal transplantation did not alter the occurrence of elevated serum troponin.Citation27 Increased concentrations were more common in patients with more advanced CKD in our study. Serum cTnT levels undergo further modifications after release, which may affect their immunogenicity and, potentially, be altered in the uremic state.Citation27 Another possible explanation is the increased cardiac damage in parallel with advanced CKD stage, which also increases serum cTnT levels.
Our study found that cTnT had significant value for LVMI and ejection fraction in pre-dialyzed patients. For CKD patients who are not receiving dialysis, however, data are limited and conflicting.Citation28,29 In uremic cardiac hypertrophy, myocardial capillary growth did not keep pace with cardiomyocyte hypertrophy.Citation30 This resulted in cardiomyocyte/capillary mismatch, increased oxygen diffusion distance, and reduced ischemic tolerance of the heart, which further increased subclinical ischemia of the myocardium and amplified the leakage of cardiac troponin across the plasma membrane of myocardial cells into the circulation.Citation31 Furthermore, increased mechanical stress altered the permeability of cardiomyocyte plasma membranes, predisposing to leakage of troponin.Citation32 Thus, the link between elevated cTnT and LVH may partly reflect leakage of this protein from hypertrophic cardiomyocyte and may signify the presence of microvascular heart disease that occurs in uremia. Using late gadolinium enhancement of cardiac magnetic resonance imaging to detect occult myocardial infarction, a recent small study found that although myocardial infarction was absent in the setting of very low cTnT, high cTnT cannot be explained solely by previous subclinical myocardial necrosis or LVH.Citation33 This leads to speculation that additional myocardial pathologies such as myocardial fibrosis may contribute to increased cTnT in patients with ESRD.
In summary, we have presented data for increased cardiac troponin concentrations that may occur early in CKD, including among a significant number of patients with moderate (stage 3) CKD and which are more common as CKD advances. The predictive value of cTnT was independent of inflammation, residual renal function, and LVH, confirming the additional value of measuring cTnT in early identified high-risk patients with ESRD.
In conclusion, increased cTnT not only shows ongoing inflammation but also is a sensitive marker of functioning renal mass in the absence of acute coronary syndrome. It is strongly correlated with factors influencing the decline in renal function; thus, it can be used as a renal risk parameter.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:16–23.
- Manjunath G, Tighiouart H, Coresh J, Macleod B. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–1129.
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169.
- Urso S, Garozzo M, Milone F, Battaglia G. Cardiovascular risk markers in hemodialysis patients. Int J Artif Organs. 2004;27:1083–1090.
- Abbas NA, John RI, Webb MC, Kempson ME. Cardiac troponins and renal function in nondialysis patients with chronic kidney disease. Clin Chem. 2005;51:2059–2066.
- Ooi DS, House AA. Cardiac troponin T in hemodialyzed patients. Clin Chem. 1995;44:1410–1416.
- Dierkes J, Domrose U, Westphal S, Ambrosch A. Cardiac troponin T predicts mortality in patients with end-stage renal disease. Circulation. 2000;102:1964–1969.
- Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation. 2002;106:2941–2945.
- Ishii J, Nomura M, Okuma T, Minagawa T. Risk stratification using serum concentrations of cardiac troponin T in patients with end-stage renal disease on chronic maintenance dialysis. Clin Chim Acta. 2001;312:69–79.
- Diris JH, Hackeng CM, Kooman JP, Pinto YM. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation. 2004;109:23–25.
- Sharma R, Gaze DC, Pellerin D. Cardiac structural and functional abnormalities in end stage renal disease patients with elevated cardiac troponin T. Heart. 2006;92:804–809.
- Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: Incidence and clinical significance. Chest. 2004;125:1877–1884.
- Lamb EJ, Wbb MC, Abbas NA. The significance of serum troponin T in patients with kidney disease: A review of the literature. Ann Clin Biochem. 2004;41:1–9.
- National Kidney Foundation. Clinical practice guidelines for chronic kidney disease: Evaluation classification and stratification. Am J Kidney Dis. 2002;39:S1–S266.
- Platt JF, Ellis JH, Rubin JM. Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: Correlation of resistive index with biopsy findings. AJR Am J Roentgenol. 1990;154:1223–1227.
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618.
- Koren MJ, Devereux RB, Casale PN, Savage DD. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352.
- Collinson PO, Hadcocks L, Foo Y, Rosalki SB. Cardiac troponins in patients with renal dysfunction. Ann Clin Biochem. 1998;35:380–386.
- Wood GN, Keevil B, Gupta J, Foley R. Serum troponin T measurement in patients with chronic renal impairment predicts survival and vascular disease: A 2 year prospective study. Nephrol Dial Transplant. 2003;18:1610–1615.
- Roppolo LP, Fitzgerald R, Dillow J, Ziegler T. A comparison of troponin T and troponin I as predictors of cardiac events in patients undergoing chronic dialysis at a veteran’s hospital: A pilot study. J Am Coll Cardiol. 1999;34:448–454.
- Goicoechea M, Garca de Vinuesa S, Gomez-Campdera F, Gutierrez MJ. Clinical significance of cardiac troponin T levels in chronic kidney disease patients: Predictive value for cardiovascular risk. Am J Kidney Dis. 2004;43:846–853.
- Zoccali C, Benedetto FA, Mallamaci F. Inflammation is associated with carotid atherosclerosis in dialysis patients. Creed investigators. Cardiovascular risk extended evaluation in dialysis patients. J Hypertens. 2000;18:1207–1213.
- Stenvinkel P. Inflammation in end-stage renal disease: The hidden enemy. Nephrology (Carlton). 2006;11:36–41.
- Lowbeer C, Stenvinkel P, Pecoits-Filho R. Elevated cardiac troponin T in predialysis patients is associated with inflammation and predicts mortality. J Intern Med. 2003;253:153–160.
- Baldus S, Koster R, Chumley P, Heitzer T. Oxypurinol improves coronary and peripheral endothelial function in patients with coronary artery disease. Free Radic Biol Med. 2005;39:1184–1190.
- Fredericks S, Chang R, Gregson H, Bewick M. Circulating cardiac troponin-T in patients before and after renal transplantation. Clin Chim Acta. 2001;310:199–203.
- Freda BJ, Tang WH, Van Lente F, Peacock WF. Cardiac troponins in renal insufficiency: Review and clinical implications. J Am Coll Cardiol. 2002;40:2065–2071.
- Vickery S, Price CP, John RI, Abbas NA. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: Relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. 2005;46:610–620.
- Madsen LH, Ladefoged S, Corell P, Schou M. N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int. 2007;71:548–554.
- Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol. 1998;9:1018–4022.
- Amann K, Ritz E. Cardiac disease in chronic uremia: Pathophysiology. Adv Ren Replace Ther. 1997;4:212–224.
- Kaye D, Pimental D, Prasad S, Maki T. Role of transiently altered sarcolemmal membrane permeability and basic fibroblast growth factor release in the hypertrophic response of adult rat ventricular myocytes to increased mechanical activity in vitro. J Clin Invest. 1996;97:281–291.
- deFilippi CR, Thorn EM, Aggarwal M, Joy A. Frequency and cause of cardiac troponin T elevation in chronic hemodialysis patients from study of cardiovascular magnetic resonance. Am J Cardiol. 2007;100:885–889.