Abstract
Objectives: Cyclosporine A (CsA) is an immunosuppressive drug, but cardiotoxicity is one of its side effects. Free oxygen radical damage and apoptosis are considered to be responsible for CsA-induced cardiotoxicity. Grape seed proanthocyanidin extract (GSPE) displays antioxidant and antiapoptotic activities. Therefore, we aimed to evaluate the effect of GSPE on CsA-induced cardiotoxicity. Materials and methods: Twenty-four rats were divided into four groups, with six rats in each group. CsA-induced nephropathy was induced by administration of 25 mg/kg CsA. The experiment was discontinued on day 21, and total oxidant system (TOS), total antioxidant system (TAS), oxidative stress index (OSI), and malondialdehyde (MDA) were measured in order to evaluate oxidative damage to the heart tissue. In addition to cardiac histopathology, transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) was performed to determine apoptosis. Results: The CsA group showed a significant increase in TOS, OSI, MDA, cardiac histopathological score, and apoptotic index (AI); in the CsA + GSPE group, OSI, MDA, cardiac histopathological score, and AI decreased significantly, and TAS levels showed a significant increase. Conclusion: In this study, we demonstrated for the first time in the literature that GSPE prevents CsA cardiotoxicity and that this effect can be achieved by antiapoptotic and antioxidant activities
INTRODUCTION
Cyclosporine A (CsA) is an immunosuppressive drug that is used after organ transplantation and in the treatment of autoimmune diseases; its specific inhibition of signal transduction pathways of T-cell receptors has resulted in its widespread use.Citation1,2 However, various organ toxicities—nephrotoxicity, cardiotoxicity, and hepatotoxicity—are its most important side effects, which restrict its long-term use.Citation1–3 There are many hypotheses to explain CsA-associated side effects. These hypotheses list the formation of free oxygen radicals, lipid peroxidation, cytochrome P450 system induction, increase in intracellular calcium, increase in vasoconstrictor eicosanoid synthesis, and apoptosis.Citation1,4–6 Free oxygen radical damage is the most frequently mentioned mechanism in the pathogenesis of CsA-associated organ toxicities.Citation1 Previously conducted studies have shown that it causes oxidative damage in the kidneysCitation1 and liverCitation7 and eventually an increase in lipid peroxidation.
The effect mechanism of CsA on heart tissue has not been fully clarified yet, and the number of available studies on this subject is very limited. However, it has been reported in recent experimental studies that CsA increases oxidative stress and causes changes in myocardial size, shape, and organization.Citation3 In addition, in some studies, besides oxidative stress, apoptosis increase is considered to be responsible in the pathogenesis of myocardial damage.Citation4
Grape seed proanthocyanidin extract (GSPE) is a biologically active polyphenolic flavonoid combination that contains oligomeric proanthocyanidin, which is a potent antioxidant abundantly found in nature.Citation8 Besides being an antioxidant and reactive oxygen species (ROS) scavenger, GSPE’s vasodilator, anticarcinogenic, antiallergic, anti-inflammatory, antibacterial, cardioprotective, immunomodulatory, antiapoptotic, and antiviral activities have been demonstrated in various experimental studies.Citation8,9 Since oxidative damage and apoptosis are blamed in the pathogenesis of CsA toxicity, we aimed to evaluate the antiapoptotic and antioxidant effects of GSPE, which have not been investigated previously. To this end, total oxidant system (TOS), total antioxidant system (TAS), oxidative stress index (OSI), and malondialdehyde (MDA) measurements were taken in heart tissue, and histopathological examinations were performed. In addition, transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) was conducted in the heart tissue to show myocardial cell apoptosis.
MATERIALS AND METHODS
A total of 24 female Sprague–Dawley rats weighing 200–250 g were used in the study. The rats were kept in cages at 22 ± 2°C with a 12 h of light/12 h of dark cycle, and they were provided with food and water according to their needs. The study was approved by the Karadeniz Technical University School of Medicine Animal Ethics Committee. The experimental animals were cared for and used in accordance with the National Institute of Health Guide.
A total of 24 rats were divided into four groups, each with six rats, after a 1-week compliance period. Throughout the study, all the rats were kept in cages in their groups of six and were fed as much standard rat feed and water as they needed.
Group 1: Control group (n = 6) gavaged with 1 cc olive oil for 21 days.
Group 2: GSPE group (n = 6) gavaged with 100 mg/kg GSPE (adjusted according to kilogram weight and mixed with olive oil to make up 1 cc) for 21 days.
Group 3: CsA group (n = 6) gavaged with 25 mg/kg CsA (adjusted according to kilogram weight and mixed with olive oil to make up 1 cc) for 21 days.
Group 4: CsA + GSPE group (n = 6) gavaged with 25 mg/kg CsA + 100 mg/kg GSPE (adjusted according to kilogram weight and mixed with olive oil to make up 1 cc) for 21 days.
On day 21, the rats were anesthetized with 90 mg/kg ketamine and 10 mg/kg xylazine, after which a midline incision was made. Then, 3 cc blood was taken for biochemical examination, and the heart tissue was rapidly removed. The rats were then exsanguinated, and the trial was terminated.
Chemicals
GSPE (0.5 mL of extract solution containing 100 mg of GSPE and 66.7 mg/g of total phenolic substance, with an oligomeric proanthocyanidin ratio of 95%) was obtained from Kale Natural Herbal Products Food, Cosmetic and Agricultural Products Co., Ltd., Edremit, Balikesir, Turkey. CsA (Sandimmun Neoral Solution®) was from Novartis (East Hanover, NJ, USA); ketamine (Ketalar®) from Pfizer (Istanbul, Turkey), and xylazine (Rompun®) from Bayer (Istanbul, Turkey).
Biochemical Analysis
CsA levels in the blood samples taken on day 21 of the study were measured by chemiluminescent microparticle immunoassay (CMI) [ARCHITECT® Cyclosporine Assay, Abbott (Fujirebio Diagnostics Inc., Malvern, PA, USA)].
The rat heart tissues were weighed and homogenized (1:10 w/v) in an ice-cold 50 mmol/L phosphate buffer (pH 7.4), using an ULTRA TURRAX T18 basic homogenizer (IKA, Wilmington, NC, USA). The homogenate was centrifuged at 4000 rpm for 5 min at 4°C, and the supernatant was used to determine MDA, TAS, and TOS levels.
Tissue MDA levels were assigned according to the method of Draper and Hadley.Citation10 Tetramethoxypropane was used as a standard, and tissue MDA levels were given as nmol/g wet tissue.
Tissue TAS, TOS, and OSI levels were assayed as previously described,Citation11 using commercial assay kits (Rel Assay Diagnostics, Gaziantep, Turkey; product no: RL0017 and RL0024, respectively). Tissue TAS levels were given as μmol Trolox Equiv./g wet tissue, and TOS levels were given as μmol H2O2 Equiv./g wet tissue. OSI values were calculated using the following formula:
Histopathological Evaluation
The specimens were fixed in a 10% formalin solution. For light microscopy, they were dehydrated using 70% and 100% alcohol solutions, processed in an autotechnicon, and embedded in paraffin. Sections 4 μm in thickness were cut with a microtome and stained with hematoxylin–eosin (HE). The stained specimens were then examined by a blinded pathologist using an Olympus BX50 (Olympus, Tokyo, Japan) light microscope. The severity of change was quantitated as none (−) to severe (+++) based on the degree of inflammation, interstitial fibrosis, and myocardial disorganization. The scoring system was as follows: (–) no damage; (+) minimal inflammatory cell infiltration, minimal focal interstitial fibrosis, and mild myocardial disorganization (<5%); (++) moderate inflammatory cell infiltration, patchy multifocal interstitial fibrosis, and moderate myocardial disorganization (5–20%); (+++) widespread inflammatory cell infiltration, severe interstitial fibrosis, and myocardial disorganization (>20%).Citation6
TUNEL Method
To label apoptotic cells, tissue samples were fixed in formalin and embedded in paraffin, and then 4-μm-thick serial sections were prepared from the tissue samples. A standard terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate nick end labeling assay (TUNEL) technique was used to detect the fragmented DNA associated with apoptosis. TUNEL staining of sections was performed using an In Situ Cell Death Detection Kit AP (Roche, Mannheim, Germany) in accordance with the manufacturer’s instructions, and the sections were then examined under light microscopy. After standard deparaffinization, the sections were hydrated and immersed in a solution of 3% hydrogen peroxidase for endogenous peroxidase blocking. The sections were incubated in a humidified chamber with the TUNEL reaction mixture. Color was then developed with diaminobenzidine tetrahydrochloride (DAB, Sigma, St. Louis, MO, USA), and the sections were counterstained with Harris hematoxylin. For the negative controls, the sections were processed in a TdT enzyme-free solution. The TUNEL staining cells appeared brown, and the nuclei of the other cells appeared blue. The stained sections were read under a light microscope at ×400 magnification. One hundred cells were counted in five microscopic fields per tissue slide. The percentage of TUNEL-positive apoptotic cells was measured, representing the apoptotic index (AI).
Statistical Analysis
Statistical analysis of data was performed using SPSS 13.0, with results expressed as mean ± SD. The groups were examined for normal distribution. Then, Kruskal–Wallis variance analysis and the Mann–Whitney U-test were used for the comparison of TOS, TAS, OSI, MDA, myocardial histopathological findings, and AI. p-Values less than 0.05 were considered to be statistically significant.
RESULTS
The initial weights of the 24 female Sprague–Dawley rats used in our study and their weights at the end of day 21 are shown in . There was no difference between the groups in terms of their initial and final weights (). The blood CsA levels at the time of the termination of the study were 1777.16 ± 783.56 ng/mL in the CsA group and 1712.43 ± 607.34 ng/mL in the CsA + GSPE group; there was no difference in CsA levels between the two groups.
Table 1. Weights and CsA levels of the groups.
The Effect of GSPE on Oxidative Damage Markers
The TOS, TAS, OSI, and MDA levels of the groups are shown in . There was a significant increase in the TAS levels of the GSPE group compared with the control group (p = 0.024). A marked increase was found in oxidative damage markers in the CsA group. TOS, OSI, and MDA values were compared with those of the control group (p = 0.004, 0.006, and 0.010, respectively). In the group receiving CsA + GSPE, the TAS levels showed an increase compared with the CsA group, while the OSI and MDA levels decreased (p = 0.045, 0.008, and 0.016, respectively). There was no difference in terms of increase in TAS values or decrease in OSI and MDA values between the CsA + GSPE group and the control group ().
Table 2. Enzyme activities of heart tissues in the control, GSPE, CsA, and CsA + GSPE groups.
The Effect of GSPE on Cardiac Histopathology Scores
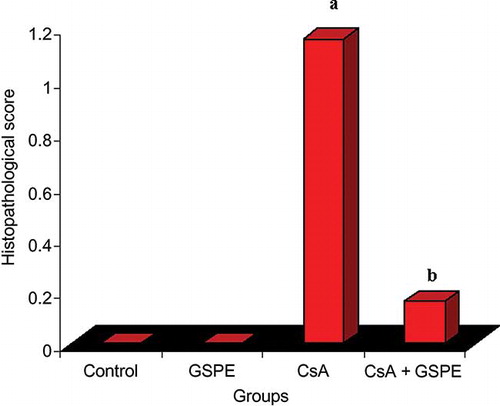
Histopathological examinations of the heart tissues did not reveal any pathological findings in the control or GSPE groups. In the CsA group, there was a distinctive myocardial inflammation and disorganization accompanied by fibrosis (). These findings were (++) in two rats, (+) in three rats, and no pathological finding in one rat. In the group receiving CsA + GSPE, interstitial fibrosis was not observed, and a marked decline was found in myocardial inflammation and disorganization. Only one rat displayed inflammation and disorganization (+), while no pathological finding was found in the other five rats (; ). In the histopathological scoring made on the basis of these findings, there was a significant difference between the CsA group and the control group (p = 0.007; ). The scores declined in the CsA + GSPE group, and the p-value was found to be 0.041 when compared with the CsA group. There was no significant difference between the CsA + GSPE group and the control group.
Figure 1. (A) Hematoxylin and eosin-stained sections (×400) of normal heart tissue (control). (B) Heart tissue of GSPE (100 mg/kg p.o.)-treated rats showing almost normal morphology. (C) Heart tissue of CsA-treated rats showing widespread myocardial edema, inflammation, disorganization, and mild fibrosis. (D) Heart tissue of GSPE (100 mg/kg p.o.) + CsA-treated rats showing prevention of CsA-induced alterations.
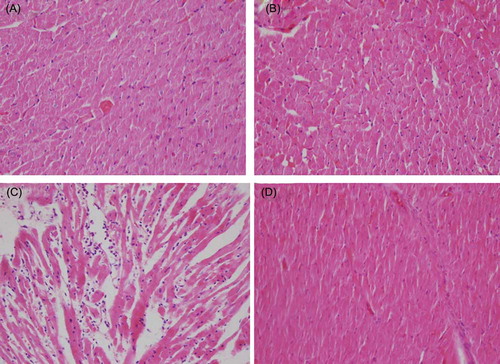
Table 3. Distribution of cardiac histopathological findings in the groups.
Figure 2. The effect of GSPE on histopathological score of myocardial cell in CsA-treated rats. The histopathological score of myocardial cell significantly increased in the CsA group compared with the control group. The histopathological score of myocardial cell significantly decreased in the CsA + GSPE group compared with the CsA group. The values are expressed as mean ± SD.
Notes: CsA, cyclosporine A; GSPE, grape seed proanthocyanidin extract. (a) CsA versus control, p = 0.007; (b) CsA + GSPE versus CsA, p = 0.041.
The Effect of GSPE on Apoptotic Index
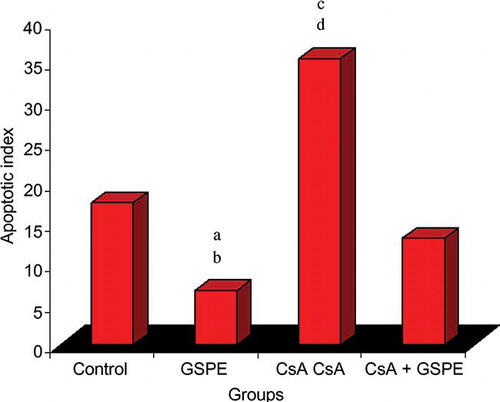
In the control, GSPE, CsA, and CsA + GSPE groups, the AI was 17.50 ± 12.94, 6.67 ± 3.61, 35.33 ± 12.24, and 13.17 ± 12.13, respectively. The AI was significantly lower in the group receiving GSPE alone compared with all the other groups, while it was the highest in the CsA group compared with all the other groups. In the group receiving CsA + GSPE, the AI decreased significantly compared with the CsA group. There was no difference between the control and GSPE groups ( and ).
Figure 3. The effect of GSPE on apoptotic index of myocardial cell in CsA-treated rats. The apoptotic index is the lowest in GSPE group and the highest in CsA group. GSPE protects the heart against chronic CsA cardiotoxicity. The values are expressed as mean ± SD.
Notes: CsA, cyclosporine A; GSPE, grape seed proanthocyanidin extract. (a) GSPE versus control, p = 0.049; (b) GSPE versus CsA, p = 0.004; (c) CsA versus control, p = 0.041; (d) CsA versus CsA + GSPE, p = 0.027.
DISCUSSION
Although there are only a few studies on the cardiotoxicity of CsA, these studies also indicate that pathogenesis is mediated by ROSCitation3,12–14 and apoptosis.Citation3,4 We showed in our study that a 21-day administration of CsA 25 mg/kg p.o. resulted in marked myocardial inflammation and disorganization accompanied by myocardial fibrosis in the CsA group. This damage was associated with oxidative damage due to the increase in TOS, OSI, and MDA; the increase in AI was thought to be mediated by apoptosis. Our findings support the available studies in the literature.Citation3,4,12–14
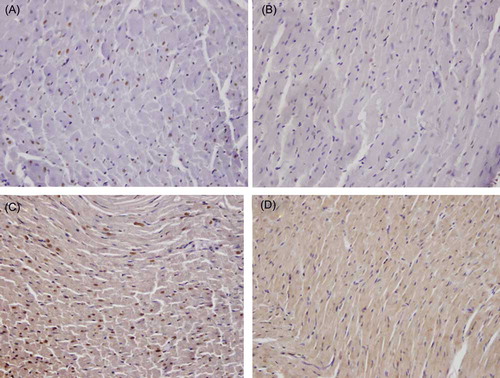
Figure 4. The effect of GSPE on apoptosis: (A) the number of TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP biotin nick end labeling)-positive myocardial cells in the control group; (B) decreased number of TUNEL-positive myocardial cells in the GSPE group; (C) increased number of TUNEL-positive myocardial cells in the CsA group; and (D) decreased number of TUNEL-positive myocardial cells in the GSPE + CsA group (TUNEL ×400).
The protective effect of a few molecules in the prevention of myocardial damage induced by CsA has been examined previously. The protective effect on oxidative damage formation was evaluated in studies conducted with melatonin,Citation14 erdosteine,Citation6 hydrocortisone,Citation12 and caffeic acid phenethyl esterCitation13; the antiapoptotic effect was also assessed in the study conducted with melatonin.Citation4 However, the literature has not included any previous studies that evaluated the effect of GSPE in preventing CsA cardiotoxicity.
It has been shown in several clinical studies that consumption of red wine decreases the frequency of cardiovascular events, a result termed the French paradox. The effect of red wine in reducing the incidence of cardiovascular events is associated with polyphenolic content, which contains proanthocyanidin and resveratrol.Citation15 The grape, from which red wine is produced, is a fruit rich in polyphenols—it contains 60–70% polyphenols.Citation16 GSPE is a biologically active polyphenolic flavonoid combination that contains oligomeric proanthocyanidin and is obtained from grape seeds.Citation8 Besides its antioxidant property, GSPE has antiapoptotic, anticarcinogenic, antiallergic, anti-inflammatory, and immunomodulatory activities, and its antioxidant activity is higher than that of vitamins E and C and β-carotene.Citation8,9,17 The cardioprotective effect of GSPE has been shown in various experimental studies, which have demonstrated that GSPE reduces myocardial damage induced by ischemia reperfusion, observed arrhythmia rate,Citation18,19 and doxorubicin-mediated cardiac damage.Citation20 Furthermore, the studies have shown that the effect of GSPE in reducing these damages was associated with its antioxidant and antiapoptotic activities.Citation18–20 In this study, we demonstrated that CsA causes myocardial damage via increased oxidative stress and myocardial cell apoptosis. GSPE markedly ameliorated this damage. The improvement in cardiac histopathology in the group receiving CsA + GSPE was associated with the effect of GSPE in improving the antioxidant system, thus decreasing oxidative damage, as well as decreasing apoptosis and CsA-associated cardiotoxicity. Our findings agree with those reported by Rezzani et al.,Citation4 who investigated the antiapoptotic and antioxidant effects of melatonin, as well as its effect in preventing CsA cardiotoxicity.
Consequently, for the first time in the literature, we showed in our study that GSPE prevents CsA cardiotoxicity. Further experimental and clinical studies conducted in light of these findings may prove GSPE, which has no toxic effects demonstrated so far, to be a promising molecule in the prevention of CsA cardiotoxicity.
ACKNOWLEDGMENTS
We thank Kale Natural Herbal Products Food, Cosmetic and Agriculture Products Co., Ltd., which provided the grape seed extract used in the study. This study was supported by the Scientific Researches Fund of Karadeniz Technical University.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Buetler TM, Cottet-Maire F, Krauskopf A, Ruegg UT. Does cyclosporin A generate free radicals? Trends Pharmacol Sci. 2000;21:288–290.
- Krejci K, Tichy T, Bachleda P, Zadrazil J. Calcineurin inhibitor-induced renal allograft nephrotoxicity. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154:297–306.
- Rezzani R. Exploring cyclosporine A-side effects and the protective role-played by antioxidants: The morphological and immunohistochemical studies. Histol Histopathol. 2006;21:301–316.
- Rezzani R, Rodella LF, Fraschini F, . Melatonin delivery in solid lipid nanoparticles: Prevention of cyclosporine A induced cardiac damage. J Pineal Res. 2009;46:255–261.
- Tang J, Wang G, Liu Y, . Cyclosporin A induces cardiomyocyte injury through calcium-sensing receptor-mediated calcium overload. Pharmazie. 2011;66:52–57.
- Selcoki Y, Uz E, Bayrak R, . The protective effect of erdosteine against cyclosporine A-induced cardiotoxicity in rats. Toxicology. 2007;239:53–59.
- Rezzani R, Buffoli B, Rodella L, Stacchiotti A, Bianchi R. Protective role of melatonin in cyclosporine A-induced oxidative stress in rat liver. Int Immunopharmacol. 2005;5:1397–1405.
- Bagchi D, Bagchi M, Stohs S, Ray SD, Sen CK, Preuss HG. Cellular protection with proanthocyanidins derived from grape seeds. Ann N Y Acad Sci. 2002;957:260–270.
- Bagchi D, Sen CK, Ray SD, . Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res. 2003;523–524:87–97.
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431.
- Harma M, Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly. 2003;133:563–566.
- Florio S, Ciarcia R, Crispino L, . Hydrocortisone has a protective effect on cyclosporin A-induced cardiotoxicity. J Cell Physiol. 2003;195:21–26.
- Rezzani R, Giugno L, Buffoli B, Bonomini F, Bianchi R. The protective effect of caffeic acid phenethyl ester against cyclosporine A-induced cardiotoxicity in rats. Toxicology. 2005;212:155–164.
- Rezzani R, Rodella LF, Bonomini F, Tengattini S, Bianchi R, Reiter RJ. Beneficial effects of melatonin in protecting against cyclosporine A-induced cardiotoxicity are receptor mediated. J Pineal Res. 2006;41:288–295.
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526.
- Nassiri-Asl M, Hosseinzadeh H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive compounds. Phytother Res. 2009;23:1197–1204.
- Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179–189.
- Sato M, Maulik G, Ray PS, Bagchi D, Das DK. Cardioprotective effects of grape seed proanthocyanidin against ischemic reperfusion injury. J Mol Cell Cardiol. 1999;31:1289–1297.
- Pataki T, Bak I, Kovacs P, Bagchi D, Das DK, Tosaki A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am J Clin Nutr. 2002;75:894–899.
- Ray SD, Patel D, Wong V, Bagchi D. In vivo protection of DNA damage associated apoptotic and necrotic cell deaths during acetaminophen-induced nephrotoxicity, amiodarone-induced lung toxicity and doxorubicin-induced cardiotoxicity by a novel IH636 grape seed proanthocyanidin extract. Res Commun Mol Pathol Pharmacol. 2000;107:137–166.
