Abstract
Frequent therapeutical use of an aminoglycoside antibiotic gentamicin (GM) is limited by its nephrotoxic effects often characterized by both morphological and functional alterations of kidney leading to acute renal failure. The aim of this study was to examine the effect of dietary calcium supplementation on GM-induced nephrotoxicity in rats. Experiments were performed on 30 adult male Wistar rats divided into three groups of 10 animals each. G-group received GM intraperitoneally at a dose of 100 mg/kg; GCa-group received the same dose of GM concomitantly with 1 g/kg calcium carbonate given orally; and C-group, serving as control, received 1 mL/day of normal saline. All groups were treated during 8 consecutive days. Quantitative evaluation of GM-induced structural and functional changes of kidney was performed by histopathological, morphometrical, and biochemical analyses. Compared with control, G-group of rats were found to have diffusely and unequally thickened glomerular basement membrane with neutrophil cells infiltration. In addition, vacuolization of cytoplasm of proximal tubule cells with coagulation-type necrosis was observed. These GM-induced pathological lesions were significantly reduced in the rats of GCa-group. Morphometric analysis revealed statistically significant differences in the size of glomeruli (area, major and minor axes, perimeter), optical density, and roundness of glomeruli (p < 0.05) between G and GCa groups. Biochemical analysis showed significant elevation in blood urea and serum creatinine concentrations, whereas potassium concentration was lowered in G-group compared with the other groups (p < 0.01). It is concluded that oral supplementation of calcium during treatment with GM resulted in significant reduction of morphological and functional kidney alterations.
INTRODUCTION
Gentamicin (GM) is an aminoglycoside antibiotic, which has long been and still is commonly used in the treatment of infections caused by Gram-negative bacilli, Enterococcus and Staphylococcus. The major adverse effects of GM are nephrotoxicity and ototoxicity. Although many hypotheses have been proposed and tested, the exact mechanisms of GM-induced nephrotoxicity still remain unclear. It has been shown that, during early time points in GM treatment (1–3 days), the antibiotic in low doses inhibits renal protein and phospholipid metabolism in rats.Citation1 Later alterations include degenerative changes (e.g., focal necrosis and apoptosis) and regenerative lesions (e.g., tubular cell proliferation and differentiation). At higher doses, GM causes alterations in the balance of certain electrolytes, impairments of mitochondrial respiration, and inhibition of protein synthesis.Citation2,3
Many studies have shown that reactive oxygen species (ROS) are involved in GM-induced renal damage. ROS directly act on cell components, including lipids, proteins, and DNA, destroying their structure. Peroxidation of membrane lipids during oxidative stress induces the fragmentation of polyunsaturated fatty acids and the release of various aldehydes and alkenes.Citation4,5 In addition, GM as a cationic amphophilic drug may cause lysosomal phospholipidosis. Inhibition of lysosomal phospholipases, subsequent accumulation of phospholipids, and the formation of lysosomal myeloid bodies have been implicated as direct mechanisms of nephrotoxicity.Citation1 Moreover, correlative confocal microscopic and electron microscopic studies suggested a role of intracellular trafficking in GM-induced nephrotoxicity.Citation6 In addition, apoptosis is an important factor of GM toxicity, which could also contribute to clear off damaged cells and control compensatory proliferative response.Citation7,8
In humans, nephrotoxicity is manifested clinically as proteinuria, enzymuria, fall in glomerular filtration rate, increase in levels of serum urea along with a slow rise in serum creatinine and hyposmolar urinary output, all leading to acute renal failure (ARF). In animals, tubular alterations have clearly been associated with the development of focal necrosis and apoptosis in the tubular epithelium, together with an extensive tubular and peritubular cell proliferation without an apparent change in kidney function.Citation2 There is a possibility that certain agents can protect the kidney from side effects of GM. Since GM-induced plasma and subcellular membrane damage appear to be critical pathogenetic events in nephrotoxicity, Ca2+ may play a protective role in this serious adverse event.Citation9 This might involve competitive displacement of Ca2+ from anionic phospholipids at the plasma and organelle membrane level, resulting in a decrease in Na–K–ATPase, adenyl cyclase, mitochondrial function and ATP production, protein synthesis, solute reabsorption, and overall cellular function. The other possibility is increase the Ca2+ solute flux, thereby competitively inhibiting the primary lesion: anionic phospholipid binding.Citation3,10 Accordingly, the main objective of the study was to examine the effect of dietary calcium loading on GM nephrotoxicity.
MATERIALS AND METHODS
In this study, 30 healthy adult male Wistar albino rats, weighing 250–300 g, were used. The animals were kept under controlled temperature (20 ± 2°C), humidity (60%), and regular light cycle (12 h light/12 h dark) conditions. They were fed with standard rat chow and allowed free access to food and water during the experiments. All experimental procedures were conducted in accordance with the principles for the care and use of laboratory animals for scientific purposes contained in the European Union regulations (Directive 2010/63/EU) and USA Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996).
Experimental Protocol
The animals were randomly divided into groups as follows: (1) Control or C-group (10 rats) received 1 mL/day 0.9% NaCl (normal saline) intraperitoneally; (2) G-group (10 rats) received GM (Galenika AD, Belgrade, Serbia) intraperitoneally at a daily dose of 100 mg/kg; (3) 10 rats in the GCa-group were treated orally with calcium carbonate (CaCO3) (Alkaloid, Skopje, Former Yugoslav Republic of Macedonia) using gastric sonde at a dose of 1.0 g/kg concomitantly with the same dose of GM applied as in the G-group. The treatment protocols were repeated during 8 consecutive days. The animals were anesthetized 24 h after the last application (9 days after the beginning of the experiment) using 80 mg/kg ketamine (Ketamidor 10%, Richter Pharma AG, Wels, Austria) and sacrificed. Blood samples for biochemical analysis were taken from the aorta (2 mL), and the kidney was subsequently removed.
Histological Analysis
After the kidneys were dissected out, tissues were fixed in 10% paraformaldehyde (in 0.1 M phosphate buffer saline) at room temperature for 48 h, then dehydrated through an ascending graded series of alcohol and embedded in paraffin wax. Tissue samples were cut at 5 μm thickness using a Histo Range Microtome (model: LKB 2218, LKB-Produkter AB, Bromma, Sweden) and stained with hematoxylin and eosin (HE) and Periodic Acid Schiff, according to conventional staining protocols. The histological sections were examined under the light microscope (Olympus BX50, Tokyo, Japan).
Morphometric Analysis
For the morphometric analysis, light microscope (Olympus BX50, Tokyo, Japan) and Micro Image 3.0 (Olympus, Tokyo, Japan) image analysis and processing software were used. Spatial calibration by object micrometer (1:100), as well as optical density calibration were performed before each analysis. The following morphometric parameters were analyzed: glomerular area, major and minor axes, perimeter, diameter, roundness, and mean optical density. Detailed description of each measured parameter was given in our previous work.Citation6 In each animal, at least 20 glomeruli were examined, excluding columns of Bertin.
Biochemical Analysis
Blood samples taken from the aorta were analyzed for markers of renal impairment. In plasma, creatinine, urea, sodium, and potassium concentrations were measured using an automatic biochemical analyzer (A25 Biosystems, Barcelona, Spain).
Statistical Analysis
The results were expressed as mean values and SD for parameters obtained during the morphometric and biochemical analyses. Statistical significance for the differences between values of morphometric and functional parameters obtained from each group was tested by the multivariate analysis of variance (MANOVA) and Student’s t-test using NCSS statistical software (NCSS Kaysville, UT, USA). In all cases, statistical significance was defined as p < 0.05.
RESULTS
Histological Analysis
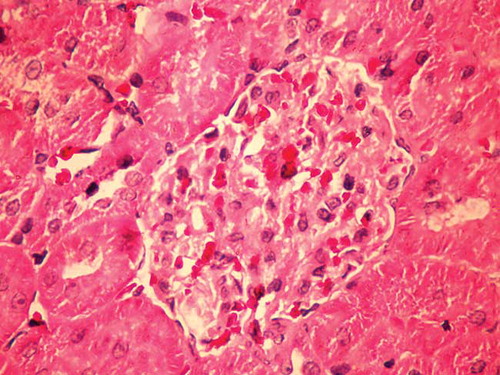
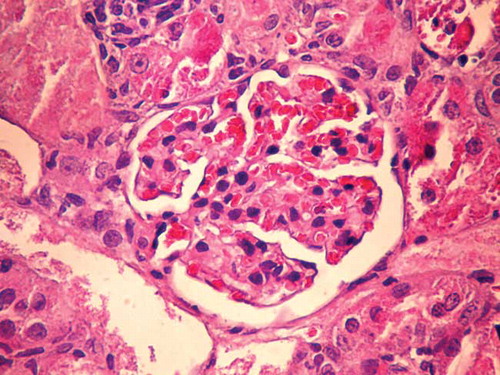
Rats in the G-group had enlarged and paler glomeruli than those in the C-group. Glomerular basement membrane was irregularly thickened with neutrophil cell infiltration. In this group of animals, areas of proximal tubule epithelial cells undergoing necrosis and apoptosis, vacuolization of cytoplasm, and epithelial desquamation were found. Distal tubules had a normal histological appearance (Figures 1 and 2).
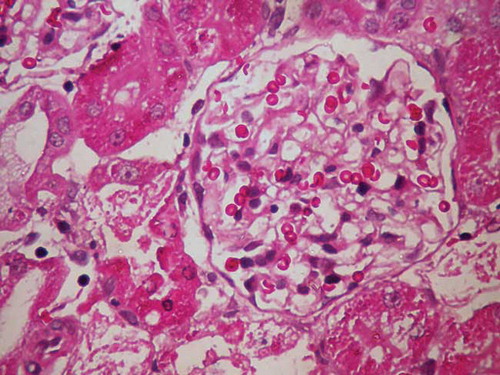
In the GCa experimental group of rats treated with GM and CaCO3, necrosis in proximal tubules was much less prevalent than in the G-group (Figure 3). In the GCa-group of rats, glomeruli were larger than those in the C-group, but were smaller than the glomeruli in the G-group of rats. Glomerular basement membrane in these animals was thinner than that in the G-group of rats. In the control group, glomerular basement membrane was thin and slender.
Morphometric Analysis
Using MANOVA test, statistically significant differences between the control and the experimental G and GCa groups were found for all glomerular morphometric parameters (Table 1). Statistically significant differences were found between the G-group of rats and the control group in the size of glomeruli (area, major axis, minor axis, average diameter, and perimeter), optical density of glomeruli (p < 0.01), and roundness of glomeruli (p < 0.05). Analysis of glomerular morphometric parameters in the GCa-group of rats did not show statistically significant differences in relation to the control group. On the other hand, significant differences were found between the experimental G and GCa groups in the size of glomeruli (area, major and minor axes, perimeter), optical density, and roundness of glomeruli (p < 0.05).
Biochemical Analysis
Analysis of biochemical parameters using MANOVA test showed significant statistical differences between the control and G and GCa groups of animals (Table 2). The multivariate statistical analysis showed significant increase in blood urea and serum creatinine concentrations in the G-group when compared with the GCa-group (p < 0.01). The concentration of potassium in the blood was significantly decreased (p < 0.01) in the G-group of animals, whereas blood concentration of sodium was decreased, but without statistical significance in comparison with the GCa-group. Blood urea and serum creatinine concentrations in the GCa-group were significantly increased compared with the control group of animals (p < 0.01).
DISCUSSION
The results of this study indicated that Ca2+ had ameliorative effect on GM nephrotoxicity as the oral loading of rats with CaCO3 exerted protective potential against renal dysfunction and structural damage caused by GM. The nephrotoxicity of GM occurs by selective accumulation of the drug in renal proximal convoluted tubules that leads to loss of its brush border integrity.Citation11 The GM nephrotoxicity involves renal free radical generation, reduction in antioxidant defense mechanisms, acute tubular necrosis, and glomerular congestion,Citation2,12–15 resulting in diminished glomerular filtration rate and renal dysfunction.Citation16 GM binds to membrane phospholipids, alters their turnover and metabolism, and, as a consequence, causes a condition known as phospholipidosis, which has been observed in humansCitation17 and experimental animals treated with the drug.Citation18,19 Lysosomal phospholipidosis results from the reduction in the available negative charge necessary for the correct function of phospholipasesCitation20 and inhibition of their A1, A2, and C1 classes.Citation21–23 Phospholipidosis correlates tightly with the level of toxicity of aminoglycosides.Citation19,24–26 Some investigators demonstrated that GM acts as an iron chelator, and that the iron–GM complex is a potent catalyst of oxygen-derived radical formation.Citation27–29 The typical clinical manifestation of aminoglycoside toxicity is nonoliguric or even polyuric renal excretion dysfunctionCitation2,30–32 accompanied by an increase in plasma creatinine, urea and other metabolic products of the organism, proteinuria, enzymuria, aminoaciduria, glycosuria, and electrolyte alterations (hypercalciuria, hypomagnesuria, hypocalcemia, and hypomagnesemia).Citation26,33,34 GM-induced nephrotoxicity is structurally associated with the occurrences of cellular desquamation, glomerular atrophy, tubular necrosis, tubular fibrosis, epithelial edema of proximal tubules, glomerular hypertrophy, perivascular edema inflammation, and glomerular congestion.Citation16,35–42 In this study, ARF was induced by a supratherapeutic dose (100 mg/kg) administration of GM. Similarly to other studies, extensive segmental necrosis, vacuolization of the tubular epithelial cells’ cytoplasm, and epithelial desquamation of proximal tubular cells were found. It is well known that GM accumulates in the lysosomes of kidney proximal tubular cells and causes apoptosis at clinically relevant doses.Citation7,43 The histopathological analysis showed that rats in the G-group had enlarged and paler glomeruli than the rats in the C-group, and the glomerular basement membrane was irregularly thickened with neutrophil cell infiltration. We also found areas of proximal tubule epithelial cells undergoing necrosis and apoptosis, vacuolization of cytoplasm, and epithelial desquamation. Histological analysis of glomeruli and proximal tubules in the experimental group of rats treated with GM and Ca2+ showed that glomeruli were larger than in the C-group, but were smaller than the glomeruli in the G-group of rats. Glomerular basement membrane in these animals was thinner than that in the G-group of rats, and proximal tubular injuries were much less prevalent than in the G-group.
Table 1. Glomerular morphometric parameters in the control group (C-group), animals treated with GM only (G-group), and animals treated with GM and CaCO3 (GCa-group).
Levels of blood urea and serum creatinine in the G-group were statistically significantly increased, whereas the values of serum sodium and potassium were decreased in comparison with the control. The values of blood urea and serum creatinine concentrations in the GCa-group were statistically significantly lower compared with the G-group. The concentration of serum potassium in the GCa-group was significantly higher compared with the G-group, whereas sodium values were increased, but without statistical significance. Remarkable elevation of urea and creatinine in the group of rats treated only with GM is an indicator of severe tubular necrosis. Morphometric analysis showed statistically significant differences between the control and the experimental G and GCa groups. Statistically significant differences were found between the G-group of rats and the control group in the size of glomeruli (area, major axis, minor axis, average diameter, and perimeter), optical density of glomeruli (p < 0.01), and roundness of glomeruli (p < 0.05). Differences were also found between the experimental G and GCa groups in the size of glomeruli (area, major and minor axes, perimeter), optical density, and roundness of glomeruli (p < 0.05). On the contrary, the glomerular morphometric parameters in the GCa-group of rats did not show statistically significant differences in relation to the control group. Oxidative stress causes Ca2+ influx into the cytoplasm from the extracellular environment and from the endoplasmic reticulum or sarcoplasmic reticulum (ER/SR) through the cell membrane and the ER/SR channels, respectively. Rising Ca2+ concentration in the cytoplasm causes Ca2+ influx into mitochondria and nuclei. In mitochondria, Ca2+ accelerates and disrupts the normal metabolism leading to cell death. In nuclei, Ca2+ modulates gene transcription and nucleases that control cell apoptosis. Both in nuclei and cytoplasm Ca2+ can regulate phosphorylation/dephosphorylation of proteins, and as a result can modulate signal transduction pathways.Citation44 Several investigators have demonstrated that calcium supplementation reduces the nephrotoxic effect likely through competitive inhibition of calcium channels in the proximal tubule.Citation9,45–47 It is suggested that high doses of GM cause wasting, and that Ca2+ loading increases the delivery of the ion to the kidney and prevents the binding of GM to the brush border membranes. It is also suggested that the mechanism of the protective action of Ca2+ may involve competitive displacement of Ca2+ from anionic phospholipids at the plasma and organelle membrane level, resulting in a decrease in Na–K–ATPase, adenyl cyclase, mitochondrial function and ATP production, protein synthesis, solute reabsorption, and overall cellular function. The other possibility is increase the Ca2+ solute flux, thereby competitively inhibiting the primary lesion: anionic phospholipid binding.Citation3,10 Another possibility may be that Ca2+ prevents critical cellular derangements induced by GM within the renal tubular cell rather than on its cell surface. Since GM has been demonstrated to induce alterations in the structure and function of a variety of subcellular membranes produced by the antibiotic,Citation48,49 prevention of subcellular membrane dysfunction by calcium may be an additional mechanism for its protective effect. The influence of dietary sodium manipulations on the protective effect of oral calcium loading suggested that Ca2+ could have a major subcellular effect in preventing GM-induced renal cell injury. Since sodium and calcium transport is directly linked to one another along the proximal tubule, volume expansion leads to the inhibition of Ca2+ transport and sodium depletion to elevations in Ca2+ transport along the proximal tubule.Citation9,50 This study indicated that Ca2+ could provide a significant protective effect against GM-induced ARF. We conclude that Ca2+ is an effective, safe, and practical agent that can reduce GM nephrotoxicity.
Table 2. Biochemical analysis of serum levels of electrolytes, blood urea, and creatinine in the control or C-group, G-group, and GCa-group of rats.
ACKNOWLEDGMENT
This work was supported by the Ministry of Education and Science Republic of Serbia grants 175092, 41018, and 43012.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Sundin DP, Sandoval R, Molitoris BA. Gentamicin inhibits renal protein and phospholipid metabolism in rats. J Am Soc Nephrol. 2001;12(1):114–123.
- Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: Nephrotoxicity. Antimicrob Agents Chemother. 1999;43(5):1003–1012.
- Ali BH. Agents ameliorating or augmenting experimental gentamicin nephrotoxicity: Some recent research. Food Chem Toxicol. 2003;41(11):1447–1452.
- Halliwell B, Chirico S. Lipid peroxidation: Its mechanism, measurement and significance. Am J Clin Nutr. 1993;57(5 Suppl.):715–725.
- Stojiljkovic N, Stoiljkovic M, Randjelovic P, Veljkovic S, Mihailovic D. Cytoprotective effect of vitamin C against gentamicin-induced acute kidney injury in rats. Exp Toxicol Pathol. 2012;64(1–2):69–74.
- Stojiljkovic N, Mihailovic D, Veljkovic S, Stoiljkovic M, Jovanovic I. Glomerular basement membrane alterations induced by gentamicin administration in rats. Exp Toxicol Pathol. 2008;60(1):69–75.
- Servais H, Jossin Y, Van Bambeke F, Tulkens PM, Mingeot-Leclercq MP. Gentamicin causes apoptosis at low concentrations in renal LLC-PK1 cells subjected to electroporation. Antimicrob Agents Chemother. 2006;50(4):1213–1221.
- Ozaki N, Matheis KA, Gamber M, . Identification of genes involved in gentamicin-induced nephrotoxicity in rats—A toxicogenomic investigation. Exp Toxicol Pathol. 2010;62(5):555–566.
- Humes HD, Sastrasinh M, Weinberg JM. Calcium is a competitive inhibitor of gentamicin-renal membrane binding interactions and dietary calcium supplementation protects against gentamicin nephrotoxicity. J Clin Invest. 1984;73(1):134–141.
- Ali BH, Al-Qarawi AA, Mousa HM. The effect of calcium load and the calcium channel blocker verapamil on gentamicin nephrotoxicity in rats. Food Chem Toxicol. 2002;40(12):1843–1847.
- Whiting PH, Brown PA. The relationship between enzymuria and kidney enzyme activities in experimental gentamicin nephrotoxicity. Ren Fail. 1996;18(6):899–909.
- Martinez-Salgado C, Lopez-Hernandez FJ, Lopez-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007;223(1):86–98.
- Elfarra AA, Duescher RJ, Sausen PJ, O’Hara TM, Cooley AJ. Methimazole protection of rats against gentamicin-induced nephrotoxicity. Can J Physiol Pharmacol. 1994;72(10):1238–1244.
- Geleilete TJ, Melo GC, Costa RS, Volpini RA, Soares TJ, Coimbra TM. Role of myofibroblasts, macrophages, transforming growth factor-beta endothelin, angiotensin-II and fibronectin in the progression of tubulointerstitial nephritis induced by gentamicin. J Nephrol. 2002;15(6):633–642.
- Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA. Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull. 2009;32(1):61–67.
- Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol Res. 2010;62(3):179–186.
- De Broe ME, Paulus GJ, Verpooten GA, . Early effects of gentamicin, tobramycin and amikacin on the human kidney. Kidney Int. 1984;25(4):643–652.
- Giuliano RA, Paulus GJ, Verpooten GA, . Recovery of cortical phospholipidosis and necrosis after acute gentamicin loading in rats. Kidney Int. 1984;26(6):838–847.
- Nonclercq D, Wrona S, Toubeau G, . Tubular injury and regeneration in the rat kidney following acute exposure to gentamicin: A time-course study. Ren Fail. 1992;14(4):507–521.
- Mingeot-Leclercq MP, Brasseur R, Schanck A. Molecular parameters involved in aminoglycoside nephrotoxicity. J Toxicol Environ Health. 1995;44(3):263–300.
- Laurent G, Carlier MB, Rollman B, van Hoof F, Tulkens P. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: In vitro and in vivo studies with gentamicin and amikacin. Biochem Pharmacol. 1982;31(23):3861–3870.
- Ramsammy LS, Josepovitz C, Lane B, Kaloyanides GJ. Effect of gentamicin on phospholipid metabolism in cultured rabbit proximal tubular cells. Am J Physiol. 1989;256(1 Pt 1):C204–C213.
- Abdel-Gayoum AA, Ali BH, Ghawarsha K, Bashir AA. Plasma lipid profile in rats with gentamicin-induced nephrotoxicity. Hum Exp Toxicol. 1993;12(5):371–375.
- Tulkens PM. Nephrotoxicity of aminoglycoside antibiotics. Toxicol Lett. 1989;46(1–3):107–123.
- Kaloyanides GJ. Drug-phospholipid interactions: Role in aminoglycoside nephrotoxicity. Ren Fail. 1992;14(3):351–357.
- Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011;79(1):33–45.
- Priuska EM, Schacht J. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochem Pharmacol. 1995;50(11):1749–1752.
- Yanagida C, Ito K, Komiya I, Horie T. Protective effect of fosfomycin on gentamicin-induced lipid peroxidation of rat renal tissue. Chem Biol Interact. 2004;148(3):139–147.
- Karakoyun B, Yuksel M, Turan P, Arbak S, Alican I. Halofuginone has a beneficial effect on gentamicin-induced acute nephrotoxicity in rats. Drug Chem Toxicol. 2009;32(4):312–318.
- Kays SE, Crowell WA, Johnson MA. Iron supplementation increases gentamicin nephrotoxicity in rats. J Nutr. 1991;121(11):1869–1875.
- Trollfors B, Alestig K, Krantz I, Norrby R. Quantitative nephrotoxicity of gentamicin in nontoxic doses. J Infect Dis. 1980;141(3):306–309.
- Klastersky J, Hensgens C, Henri A, Daneau D. Comparative clinical study of tobramycin and gentamicin. Antimicrob Agents Chemother. 1974;5(2):133–138.
- Parsons PP, Garland HO, Harpur ES, Old S. Acute gentamicin-induced hypercalciuria and hypermagnesiuria in the rat: Dose-response relationship and role of renal tubular injury. Br J Pharmacol. 1997;122(3):570–576.
- Banday AA, Farooq N, Priyamvada S, Yusufi AN, Khan F. Time dependent effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissues. Life Sci. 2008;82(9–10):450–459.
- Bledsoe G, Crickman S, Mao J, . Kallikrein/kinin protects against gentamicin-induced nephrotoxicity by inhibition of inflammation and apoptosis. Nephrol Dial Transplant. 2006;21(3):624–633.
- Abdel-Naim AB, Abdel-Wahab MH, Attia FF. Protective effects of vitamin E and probucol against gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 1999;40(2):183–187.
- Shirwaikar A, Malini S, Kumari SC. Protective effect of Pongamia pinnata flowers against cisplatin and gentamicin-induced nephrotoxicity in rats. Ind J Exp Biol. 2003;41(1):58–62.
- Atessahin A, Karahan I, Yilmaz S, Ceribasi AO, Princci I. The effect of manganese chloride on gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 2003;48(6):637–642.
- Polat A, Parlakpinar H, Tasdemir S, . Protective role of aminoguanidine on gentamicin-induced acute renal failure in rats. Acta Histochem. 2006;108(5):365–371.
- Ozbek E, Cekmen M, Ilbey YO, Simsek A, Polat EC, Somay A. Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF kappaB pathways. Ren Fail. 2009;31(5):382–392.
- Abdel-Raheem IT, El-Sherbiny GA, Taye A. Green tea ameliorates renal oxidative damage induced by gentamicin in rats. Pak J Pharm Sci. 2010;23(1):21–28.
- Lakshmi BVS, Sudhakar M. Protective effect of zingiber officinale on gentamicin-induced nephrotoxicity in rats. Int J Pharmacol. 2010;6(1):58–62.
- Stojiljkovic N, Veljkovic S, Mihailovic D, . Protective effects of pentoxifylline treatment on gentamicin-induced nephrotoxicity in rats. Ren Fail. 2009;31(1):54–61.
- Ermak G, Davies KJ. Calcium and oxidative stress: From cell signaling to cell death. Mol Immunol. 2001;38(10):713–721.
- Bennett WM, Elliott WC, Houghton DC, Gilbert DN, DeFehr J, McCarron DA. Reduction of experimental gentamicin nephrotoxicity in rats by dietary calcium loading. Antimicrob Agents Chemother. 1982;22(3):508–512.
- Perez de la Cruz MJ, Cadorniga R, Ochoa MC, Albarran I, Herrero-Vanrell R, Pastoriza P. Chronopharmacokinetics and calcium in the prevention of gentamicin-induced nephrotoxicity in rabbits. Biopharm Drug Dispos. 1998;19(6):407–412.
- Pannu N, Nadim MK. An overview of drug-induced acute kidney injury. Crit Care Med. 2008;36(4 Suppl.):S216–S223.
- Weinberg JM, Humes HD. Mechanisms of gentamicin-induced dysfunction of renal cortical mitochondria. Effects on mitochondrial respiration. Arch Biochem Biophys. 1980;205(1):222–231.
- Knauss TC, Weinberg JM, Humes HD. Alterations in renal cortical phospholipid content induced by gentamicin: Time course, specificity and subcellular localization. Am J Physiol. 1983;244(5):F535–F546.
- Goldberg M, Agus ZS, Goldfarb S. Renal handling of phosphate, calcium, and magnesium. In: Brenner BM, Rector FC, eds. The Kidney. Philadelphia, PA: WB Saunders Co.; 1976:344–390.