Abstract
Aim: To investigate the correlation of the functional disequilibrium of regulatory T cells (Treg)/T-helper (Th17) cells with calcification and to explore the significance of their influence on the outcome of cardiovascular disease (CVD) in uremic patients after hemodialysis (HD). Methods: Out of 66 uremia patients, 36 patients had CVD after HD (maintenance hemodialysis (MHD) group1) and 30 patients did not have CVD (MHD group2). Twenty healthy volunteers were selected as normal control group. Peripheral blood mononuclear cells were isolated and treated with recombinant human bone morphogenetic protein-2 (rhBMP-2). Treg and Th17 frequencies were measured by flow cytometry. Forkhead/winged helix transcription factor (Foxp3) and retinoic acid receptor-related orphan receptor-γt (ROR-γt) mRNA expressions were measured by real-time quantitative polymerase chain reaction. Levels of interleukin (IL)-10 and IL-17 were detected by enzyme-linked immunosorbent assay. Results: When compared with controls, rhBMP-2 upregulates Treg/Th17 functional disequilibrium in uremia patients, displaying higher Treg and Th17 frequencies, Foxp3 and ROR-γt expressions, and levels of cytokines (p < 0.05). These differences were also significant between MHD group1 and group2 (p < 0.05). It was also observed that Treg/Th17 functional disequilibrium was not only correlated with a calcification state but also consistent with the CVD. Conclusion: The Treg/Th17 cell function disequilibrium might act synergistically with calcification in the high incidence of CVD after HD.
INTRODUCTION
Hemodialysis (HD) provides effective clearance of small-molecule toxins and is thus an effective treatment strategy to prolong survival in uremia patients. Mortality of uremia patients treated with HD remains high, mainly due to cardiovascular disease (CVD accounts for 40–50% of all deaths) resulting from accelerated atherosclerosis (AS).Citation1,2 Vascular calcification is not only the main pathological process contributing to uremia patients complicated by AS, but it is also an independent risk factor for increased mortality and poor prognosis of uremia patients treated by HD.Citation3 Furthermore, our previous study demonstrated that the imbalance of regulatory T cells (Treg)/ T-helper (Th17) cells acts synergistically with inflammation on immune-mediated AS in uremia patients on maintenance hemodialysis (MHD),Citation4 but the pathogenic mechanisms are still unclear. Therefore, we presume that functional disequilibrium of Treg/Th17 cells may act synergistically with calcification in increasing the incidence of CVD after HD. To explore this possibility, we enrolled uremia patients on HD with and without CVD and healthy volunteers as study subjects. All uremia patients had no history of CVD before HD. Our present study had the following objectives: (1) to determine whether Treg/Th17 functional disequilibrium exists in uremia patients on HD; (2) to determine the correlation between the Treg/Th17 functional disequilibrium and CVD in uremia patients on HD; and (3) to investigate the relationship between Treg/Th17 functional disequilibrium and calcification in uremia patients on HD. To avoid the potential confounding influences of the type of HD on results, we examined patients treated by HD employing the same type of dialyzer membrane and the same dialysis mode.
PATIENTS AND METHODS
Patients
We studied a total of 66 uremia patients (40 males and 26 females). They were on HD for at least 3 months. The etiologies of 66 uremia patients in the study population were chronic glomerulonephritis (n = 26, 39%), nephrosclerosis (n = 16, 24%), diabetic nephropathy (n = 10, 15%), polycystic kidney disease (n = 4, 6%), chronic interstitial nephritis (n = 6, 9%), or uremia of unknown etiology (n = 4, 6%). All patients were hemodialyzed for 4–5 h after every 2–3 days through internal arteriovenous fistula of the upper limb with blood flow at 200–250 mL/min. Patients were dialyzed using a bicarbonate dialysate and a polysulfone membrane dialyzer flowing at 500 mL/min. Sixty-six patients were divided into two groups according to their histories of CVD. Thirty-six patients who suffered from CVD were grouped into MHD group1 and the remaining 30 patients who did not suffer were grouped into MHD group 2. CVD included unstable angina pectoris (n = 6, 17%), severe arrhythmia (n = 14, 39%), left ventricular (LV) hypertrophy (n = 10, 28%), cerebral vascular accidents (n = 2, 5%), and congestive heart failure (n = 4, 11%) as evidenced mainly by electrocardiographs and echocardiograms. The normal control group consisted of 20 healthy volunteers (10 men and 10 women) with no known coronary AS risk factors and a mean age of 44.00 ± 9.00 years. This study conformed to all institutional guidelines for human studies. Informed consent was obtained from each patient and the patients were stable and had HD regularly. No patient was being treated with anti-inflammatory drugs, none had any diagnosed autoimmune disease, malignant disease, advanced liver disease, infection, thromboembolism, disseminated intravascular coagulation, or valvular heart disease, and none of the patient was using a pacemaker. Patients were negative for hepatitis B, hepatitis C, and HIV. Additional information regarding the study subjects is shown in .
Table 1. Basic clinical and laboratory characteristics of the study population.
Preparation of Peripheral Blood Mononuclear Cells (PBMCs) and Cell Culture
Blood samples (50 mL) of patients were drawn into heparinized tubes after an 8-h fast. peripheral blood mononuclear cells (PBMCs) were separated using Ficoll density gradient centrifugation with lymphocyte separation medium.Citation5 The enriched PBMCs were recovered, washed twice in phosphate-buffered saline (PBS), counted using Trypan blue, and resuspended at 3 × 106 cells/mL in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mmol/L glutamine, and 10% heat-inactivated fetal calf serum (Gibco BRL, Gaithersburg, MD, USA). With or without 100 ng/mL recombinant human bone morphogenetic protein-2 (rhBMP-2) were in cell culture for 24 h.
Flow Cytometric Analysis of Treg and Th17
To measure Th17 cell frequency, the PBMCs suspension was incubated with phycoerythrin (PE)-conjugated antihuman CD4 at 4°C for 20 min, and then fixed, permeabilized, and stained with fluorescein isothiocyanate (FITC)-conjugated antihuman interleukin (IL)-17 according to the operating instruction. To detect Treg cells, the PBMCs were incubated with peridinin chlorophyll protein (PerCP)-conjugated antihuman CD4 and FITC-conjugated antihuman CD25 for surface staining. After fixation and permeabilization, the cells were stained with PE-conjugated antihuman Foxp3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The cells were resuspended after washing with PBS and analyzed using a FAC Scan cytometer equipped with CellQuest software (BD Biosciences Pharmingen, San Diego, CA, USA). Isotype controls were used as compensation controls and to confirm antibody specificity.
Detection of Foxp3 and ROR-γt by Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted with Trizol (TaKaRa Bio Inc., Kyoto, Japan) according to the manufacturer’s instructions. For real-time quantitative polymerase chain reaction (RT-qPCR), cDNA was synthesized using primescript RT-PCR kit (TaKaRa Bio Inc.,). The following primers (Shanghai Biotechnology Company, Shanghai, China) were used to detect human Foxp3, retinoic acid receptor-related orphan receptor-γt (ROR-γt), and β-actin (internal control): Foxp3 forward 5′-CATCTGTGGCATCATCCGAC-3′ and reverse 5′-GAGCGTGGCGTAGGTGAAAG-3′; ROR-γt forward 5′-GCCTACAATGCTGACAACCG-3′ and reverse 5′-GGATGCTTTGGCGATGAGTC-3′; and β-actin forward 5′-TGACGTGGACATCCGCAAAG-3′ and reverse 5′-CTGGAAGGTGGACAGCGAGG-3′. All three cDNAs were amplified using the following thermal cycle: denaturation at 94°C for 4 min, followed by 35 cycles of denaturation at 94°C for 20 s, annealing at 60°C for 30 s, polymerization at 72°C for 30 s, and brief detection at 72°C. The signal was expressed relative to β-actin (relative ratio = average copy number of target gene in the sample/average copy number of β-actin).
Detection of IL-10 and IL-17 levels by Enzyme-Linked Immunosorbent Assay
The levels of IL-10 and IL-17 were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D, Minneapolis, MN, USA). Intra-assay and interassay coefficients of variation for all ELISA readings were less than 10%. All samples were measured in duplicate.
Statistical Analysis
All data were analyzed using SPSS v17.0 software (SPSS, Chicago, IL, USA). For unpaired data, statistical differences were determined using unpaired Student’s t-test if the variances were non-homogenous. Paired observations were analyzed using paired t-test or one-way repeated measures analysis of variance (ANOVA). A p-value of <0.05 was considered to be statistically significant. Values are expressed as mean ± SD in the text and figure.
RESULTS
Basic Clinical Characteristics of Uremia Patients
As shown in , there were no significant differences in age, gender, risk factors, and medications between uremic groups. Neither blood urea nitrogen nor serum creatinine was significantly different between MHD group1 and group2 (p > 0.05).
Assessment of Heart Function in Subgroup of Uremia Patients on HD
Uremia patients in the subgroup New York Heart Association (NYHA) III–IV experienced significant LV dilatation and systolic dysfunction when compared with the subgroup NYHA I–II demonstrated by an increased LV end-diastolic diameter (LVEDd), reduced ejection fraction, fractional shortening, and LV mass index (). In addition, the NYHA III–IV group exhibited an increase in the frequency ratio of Treg:Th17 as compared to the NYHA I–II group (6.9:46.5 vs. 4.6:28.6; p < 0.05).
Table 2. Assessment of heart function in the subgroup of uremia patients on HD.
Analysis of Treg and Th17 Cell Frequencies among Groups
As shown in and , MHD patients had higher Treg and Th17 cell frequencies but lower frequency ratio of Treg and Th17 than healthy volunteers (p < 0.05). Among MHD patients, no significant differences in the Treg cell frequencies were found in MHD group1 and group2 (p > 0.05), while Th17 cell frequencies were significantly higher in the MHD group1 than group2 (p < 0.05). Consequently, MHD group1 had lower frequency ratio of Treg and Th17 than group2 (p < 0.05). All pair-wise inter-the same subgroup comparisons reveal that rhBMP-2 upregulates the Treg cell frequencies (p > 0.05) and the Th17 cell frequencies (p < 0.05); rhBMP-2 downregulates the frequency ratio of Treg and Th17 either in MHD patients or in healthy volunteers. However, the downregulation of frequency ratio of Treg and Th17 was not significant in healthy volunteers (p > 0.05).
Figure 1. Frequency of the Treg and Th17 cells among groups. The representative photographs of flow cytometry analysis of Treg and Th17 cell surface expression and intracellular staining are without and with rhBP-2 from MHD group1 (A and D, respectively), MHD group2 (B and E, respectively), and normal control group (C and F, respectively).
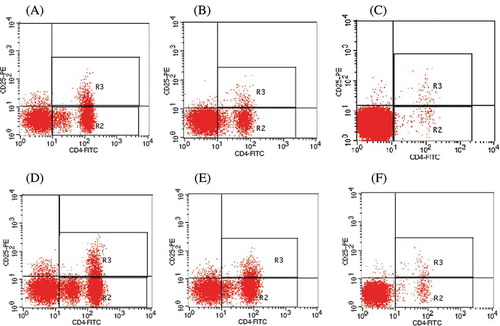
Table 3. Comparison of Treg and Th17 cell frequencies among groups.
mRNA Expressions of Foxp3 and ROR-γt
MHD patients had higher Foxp3 and ROR-γt expressions but lower expression ratio of Foxp3 and ROR-γt than healthy volunteers (p < 0.05). Among MHD patients, no significant differences in the Foxp3 expressions were found in MHD group1 and group2 (p > 0.05), while ROR-γt expressions were higher in the MHD group1 than group2 (p < 0.05). Consequently, MHD group1 had lower expression ratio of Foxp3 and ROR-γt than group2 (p < 0.05). All pair-wise inter-the same subgroup comparisons reveal that rhBMP-2 upregulates the Foxp3 expressions (p > 0.05) and ROR-γt expressions (p < 0.05) but downregulates the expression ratio of Foxp3 and ROR-γt either in MHD patients or in healthy volunteers. However, the upregulation of ROR-γt expressions and the downregulation of expression ratio of Foxp3 and ROR-γt were not significant in healthy volunteers (p > 0.05) ().
Table 4. mRNA expression of Foxp3 and ROR-γt among groups.
Table 5. Levels of IL-10 and IL-17 among groups.
Levels of Treg and Th17 Cell-Related Cytokines among Groups
When compared with healthy volunteers, levels of IL-10 and IL-17 were higher in MHD patients (p < 0.05). Among MHD groups, IL-10 and IL-17 levels were higher in the MHD group1 than group2, while the IL-10 levels were not significantly different between MHD groups (p > 0.05). All pair-wise inter-the same subgroup comparisons reveal that rhBMP-2 upregulates the IL-10 levels (p > 0.05) and IL-17 levels (p < 0.05) in MHD patients. However, the upregulation of IL-10 and IL-17 levels was not significant in healthy volunteers (p > 0.05) ().
DISCUSSION
Epidemiological studies suggest that the mortality of uremia patients treated with HD remains high, mainly due to AS and concomitant CVD. In essence, these cardiovascular complications are mainly chronic autoimmune-response diseases, including innate and adaptive immunity diseases and autoimmune reaction diseases. They require the contribution of a variety of immune cells, especially T lymphocytes.Citation6
Treg and Th17 cells are both CD4+ T cells while distinct from Th1 and Th2 cells. They are generated from a common precursor but are functionally antagonistic.Citation7 The Treg-specific transcription factor forkhead/winged helix transcription factor (Foxp3) is critical for anti-inflammatory responses and for maintaining immune tolerance mainly by regulating the secretion of the anti-inflammatory cytokines IL-10.Citation8 Recent studies have shown that the progression of CVD, like other autoimmunity inflammatory reactions, is modulated by the Treg cells.Citation9 In contrast, the Th17 cells expressing ROR-γt play a crucial role in the induction of autoimmune tissue injuries and inflammation mainly by producing inflammatory cytokine IL-17.Citation10,11 Animal experiments indicated that the levels of IL-17 at AS foci were positively correlated with the degree of AS.Citation12,13 Moreover, the Treg/Th17 imbalance markedly impacts the proinflammatory and anti-inflammatory balance, which has been demonstrated in acute coronary syndrome patients and plays a potential role in the initiation and process of CVD.Citation14
The prime objective of this study was to assess Treg/Th17 functional equilibrium in uremia. Our results demonstrated that uremia patients exhibited functional disturbances in immune cell populations. Compared to healthy controls, frequencies of Treg and Th17 cells, expressions of transcription factor Foxp3 and ROR-γt, Treg-related cytokines (IL-10), and Th17-related cytokines (IL-17) were found to be increased in uremia patients. On the other hand, the frequency ratio of Treg and Th17 and expression ratio of Foxp3 and ROR-γt were found to be decreased. It appears that uremia patients with a history of CVD after treatment with HD (MHD group1) had greater Treg/Th17 functional disequilibrium than in patients without a history of CVD (MHD group2). This disequilibrium was found to be induced not only by uremia independent of dialysis membrane type and dialysate, but also by severity of calcification.
The secondary objective of this study was to investigate the relationship between Treg/Th17 functional disequilibrium and CVD in uremia patients after HD. The subgroup analysis of uremia patients on MHD revealed higher frequencies of Th17 cells but lower frequencies of Treg cells in patients with heart function class NYHA III–IV than in patients with NYHA I–II. A Treg/Th17 cell disequilibrium was found to correlate with the onset of CVD. Moreover, the patients with a greater Treg/Th17 disequilibrium had more severe heart failures. We suggest that the Treg cell downregulation and Th17 cell upregulation may lead to myocardial dysfunction through immune-mediated myocardial injury in uremia patients. Therefore, immunotherapy with the goal of decreasing Treg/Th17 disequilibrium may have a protective effect in uremia patients.
It is known that vascular calcification is not only a serious event during the final stage of nephropathy, but also an independent risk factor in the death and poor prognosis of patients on HD.Citation3,15 Epidemiological studies have also demonstrated that 54–100% (average 83%) of patients with chronic renal failure show different degrees of vascular calcification. In fact, the greater the vascular calcification is, the higher the fatality rate is.Citation16,17 Therefore, the third question addressed by this study was whether the Treg/Th17 functional disequilibrium in uremia patients on HD was associated with a calcification state.
Bone morphogenetic protein-2 (BMP-2) is regarded as a major activator of calcification. It is a member of the TGF-β superfamily and is a potent inducer of bone growth and proliferation and differentiation of bone cells. In addition to bone tissue, BMP-2 is expressed in both developing aorta and calcified atherosclerotic endotheliocytes that have been injured.Citation18 BMP-2 increases the degree of calcification and bone differentiation in calcigerous cells cultured in vitro. Moreover, it counteracts the inhibitory effects of endothelial cells on the formation of calcified tubercle from calcigerous cell in vitro. Our study found that the downregulation of rhBMP-2 on the frequency ratio of Treg and Th17 and the expression ratio of Foxp3 and ROR-γt was not significant in healthy controls. The downregulation degree of rhBMP-2 in uremia patients was significantly higher than in healthy controls. On the other hand, the Treg and Th17 cell frequencies, Foxp3 and ROR-γt expressions, and IL-10 and IL-17 levels were upregulated significantly by rhBMP-2. The upregulation degree of rhBMP-2 in uremia patients was also significantly higher than in healthy controls.
In conclusion, our results indicate that a Treg/Th17 functional disequilibrium exists in uremia patients and is associated with the calcification state and the development of CVD. It was reported that BMP-2 has a high affinity for the bone morphogenetic protein receptor (BMPR)-I receptor subtype and initiates signal transduction after BMPR-I combines with BMPR-II to form a heterodimer.Citation19 The activation of signal transduction pathways depends on the distinct combinations of receptors bound by BMP-2.Citation20 Binding of BMP-2 promotes vascular calcification mainly through the BMP-2-cbfα1 signal pathwayCitation21 or BMP-2-MSX2 signal pathway.Citation22 Further efforts should be made to identify the precise mechanism of Treg/Th17 functional disequilibrium and calcification in uremia patients on HD. The Treg/Th17 cell functional equilibrium will provide new therapeutic targets for the prevention and treatment of uremia patients on HD complicated by CVD.
ACKNOWLEDGMENTS
We thank Prof. Weixue Tang for technical assistance. This study was sponsored by Medicine Scientific Research Project of Chongqing Health Bureau (No. 2010-01-16).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Xuwu G, Chupeng C, Yizhong W. The clinical significance of plasma N-terminal brain natriuretic peptide level in chronic kidney disease patients accompanied by heart failure without dialysis. Hebei Med. 2009;15(9):1015–1018.
- Sherer Y, Shoenfeld Y. Mechanisms of disease: Atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol. 2006;2(2):99–106.
- DeLoach SS, Townsend RR. Vascular stiffness: Its measurement and significance for epidemiologic and outcome studies. Clin J Am Soc Nephrol. 2008;3(1):184–192.
- Zhang J, Hua G, Zhang X, . Regulatory T cells/T-helper cell 17 functional imbalance in uremic patients on maintenance hemodialysis: A pivotal link between microinflammation and adverse cardiovascular events. Nephrology. 2010;15(1):33–41.
- Sharma UK, Song HF, Willingham FF, . Diagnosis of human immunodeficiency virus infection using citrated whole blood. Clin Diagn Lab Immunol. 1997;4(3):261–263.
- Carrero JJ, Yilmaz MI, Lindholm B, . Cytokine dysregulation in chronic kidney disease: How can we treat it? Blood Purif. 2008;26(3):291–299.
- Mucida D, Park Y, Kim G, . Reciprocal Th17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260.
- Sakaguchi S, Ono M, Setoguchi R, . Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212(1):8–27.
- Mor A, Planer D, Luboshits G, Afek A, . Role of naturally occurring CD4+CD25+ regulatory T cell in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(4):893–900.
- Csiszar A, Ungvari Z. Synergistic effects of vascular IL-17 and TNF-a may promote coronary artery disease. Med Hypotheses. 2004;63(4):696–698.
- Abdulahad WH, Stegeman CA, Limburg PC, . Skewed distribution of Th17 lymphocytes in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2008;58(7):2196–2205.
- de Boer OJ, van der Meer JJ, Teeling P, . Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J Pathol. 2009;220(4):499–508.
- Chen S, Crother TR, Arditi M. Emerging role of IL-17 in atherosclerosis. J Innate Immun. 2010;2(4):325–333.
- Cheng X, Yu X, Ding Y, . The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127(1):89–97.
- Covic A, Kanbay M, Voroneanu L, . Vascular calcification in chronic kidney disease. Clin Sci. 2010;119(3):111–121.
- Negri AL. Vascular calcification in chronic kidney disease: Are these new treatments? Curr Vasc Pharmacol. 2005;3(2):181–184.
- Toussaint ND, Lau KK, Strauss BJ, . Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant. 2008;23(2):586–593.
- Csiszar A, Smith KE, Koller A, . Regulation of bone morphogenetic protein-2 expression in endothelial cells: Role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111(18):2364–2372.
- Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97(2):105–114.
- Hauburger A, von Einem S, Schwaerzer GK, . The pro-form of BMP-2 interferes with BMP-2 signalling by competing with BMP-2 for IA receptor binding. FEBS J. 2009;276(21):6386–6398.
- Liao XB, Zhou XM, Li JM, . Taurine inhibits osteoblastic differentiation of vascular smooth muscle cells via the ERK pathway. Amino Acids. 2008;34(4):525–530.
- Al-Aly Z, Shao JS, Lai CF, . Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2589–2596.
