Abstract
Background: Disorders of mineral metabolism can facilitate the progression of vascular calcification and are closely associated with adverse outcomes in end-stage renal disease (ESRD). miR-15b has been implicated in the epigenetic regulation of key metabolism, stress response, and osteoblast differentiation. Methods and results: In this study, we detected miR-15b in the plasma of 30 patients with ESRD and 20 healthy controls using real-time quantitative RT-PCR (RT-qPCR). Compared with healthy controls, the circulating levels of miR-15b were significantly reduced in patients with ESRD. However, there is no significant difference in circulating miR-15b levels when comparing prehemodialysis and posthemodialysis in patients with ESRD. In addition, to further estimate the potential roles of aberrantly expressed candidate miR-15b in the pathogenesis of ESRD, we utilized a bioinformatics exploratory analysis and identified gene ontology “biological process” classifications which revealed that dysregulated circulating miR-15b might be involved in phosphate metabolism. Furthermore, circulating miR-15b positively correlated with both estimated glomerular filtration rate (r = 0.502, p = 0.003) and hemoglobin levels (r = 0.432, p = 0.017) and inversely correlated with phosphate level (r = −0.516, p = 0.004). Conclusion: The findings indicated that the dysregulated miR-15b might contribute to the progression of ESRD by modulating genes that might be involved in the phosphate metabolism, which might have the potential of being used as a biomarker for monitoring disease.
INTRODUCTION
MicroRNAs (miRNAs) are a class of small (≈22-nucleotide) noncoding RNAs that modulate gene expression at the posttranscriptional level. They bind to a complementary site in the targeted transcript’s 3′ untranslated region, leading either to translational repression or to degradation of the message RNA.Citation1 miRNAs regulate various cellular processes and are critically involved in many physiologic and pathologic processes in health and diseases, including kidney diseases.Citation2 miRNAs have been shown to play essential roles in maintaining renin cells and in the function of the kidneyCitation3 and might lead to common disease complications. Aberrant expression patterns of miRNAs have been described in diabetic nephropathy, polycystic kidney disease, and kidney allograft rejection.Citation4,5
miRNAs were previously considered to act as intracellular miRNAs to modulate gene expression. However, accumulating evidence has demonstrated that miRNAs are remarkably stable in the blood, can withstand repetitive freezing/thawing cycles, and circulate within membrane-bound vesicles that are resistant to RNase digestion. A recent study additionally demonstrated that circulating Argonaut 2 complexes, carrying a number of miRNAs independent of vesicles, are a mechanism responsible for the stability of circulating miRNAs.Citation6 Likewise, differently expressed miRNA profiles have been described in the blood of cases with cardiovascular disease (CVD), including coronary artery disease, myocardial infarction, and heart failure.Citation7–9 Recent studies demonstrated that in the circulation, specific miRNAs can serve as potential biomarkers of various diseases. Moreover, detection of circulating miRNAs can provide important novel information to assist in diagnosing and monitoring disease.
The CVD is the primary cause of death in patients with end-stage renal disease (ESRD). The higher risk seen in hemodialysis patients cannot be completely elucidated by traditional risk factors; therefore, more emphasis has been put on novel risk factors, including anemia, abnormal calcium, phosphate metabolism, and vascular calcification, proposing to explain the higher risk of CVD.Citation10 Remarkably, vascular calcification has been demonstrated to increase the morbidity and mortality of CVD in patients with ESRD. Also, a disturbance in mineral metabolism, predominantly hyperphosphatemia, has been shown to facilitate the progression of vascular calcification. Several studies have recognized miRNAs as important mediators in the pathological process of vascular calcification.Citation11,12 To date, no specific miRNAs have been identified to be associated with mineral metabolism in ESRD. miR-15b has emerged as the key regulator of various biological processes including key metabolism, cell division, and osteoblast differentiation.Citation13,14 In this study, we aim to detect the circulating level of miR-15b in patients with ESRD receiving maintenance hemodialysis, which is expected to help provide new insights into the development of ESRD and the manifestation of complications in ESRD.
MATERIALS AND METHODS
Patients and Volunteers
Eligible patients were those with ≥18 years of age and with ESRD who have been receiving maintenance hemodialysis 3 times weekly for at least 3 months before screening. The primary disease of patients with ESRD was chronic glomerulonephritis that was proved by biopsy. Twenty healthy adults without any evidence of CKD or inflammatory disorders served as the control group. All participating subjects gave their written informed consent before entering the study. All study protocols and consent forms were approved by the second clinical medical college (Shenzhen People’s Hospital) of Jinan University, which abides by the Helsinki Declaration on ethical principles for medical research involving human subjects.
Blood Processing
Whole blood (2.5 mL) from subjects was collected via a direct venous puncture into tubes containing sodium citrate (BD Biosciences, San Jose, CA, USA). All blood was processed for isolation of plasma within 4 h of collection. Blood was processed by spinning at 2000 × g for 10 min at room temperature. The plasma was transferred to RNase-free tubers and stored at –80°C.
RNA Isolation
So far, no reliable endogenous control miRNA has been established and confirmed to normalize differences in the miRNA content. Synthetic cel-lin-4 (Shanghai GenePharma, Shanghai, China) was spiked in as controls, RNA was isolated by using a TRIzol-based miRNA isolation protocol, and 300 μL plasma was mixed with 1200 μL TRIzol (Invitrogen, Carlsbad, CA, USA) and subsequently mixed with 200 μL chloroform. The organic and aqueous phases were separated by centrifugation. The aqueous phase containing RNA was carefully removed and RNA was precipitated by the addition of 10 μL SiO2 and 400 μL 75% ethanol, respectively. The RNA was eluted by adding 25 μL RNase-free water. In this study, we have suggested the use of lin-4 for the normalization of RNA preparation.
Real-Time Quantitative RT-PCR of Mature miRNAs and Data Analysis
To determine the circulating levels of miRNAs, we performed real-time quantitative RT-PCR (RT-qPCR) using RNA isolated from n = 20 healthy controls and n = 30 patients before and after hemodialysis. Target-specific stem-loop RT primer bound to the 3′ portion of miRNA molecules and was reverse transcribed with M-MLV Reverse Transcriptase (Promega, Madison, WI, USA). Then, real-time PCR was performed on an ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with miRNA-specific forward primer and reverse primer. Each SYBR Green reaction was performed with 5μL template cDNA, 10 μL 2 × SYBR Greens PCR Master Mix (TOYOBO, Tokyo, Japan), 0.5 μL of each primer, and water to adjust the final volume to 20 μL. The miRNA-specific forward primer sequences were designed based on the mature miRNA sequences obtained from the miRBase database (http://www.mirbase.org/). The RT primers used are (1) the RT forward: hsa-miR-15b: 5′-ACACTCCAGCTGGGTAGCAGCACATCATGGTTTAcel-lin-4: 5′-ACACTCCAGCTGGGTCCCTGAGACCTCAAGTG and (2) miRNAs reversed: CTCAACTGGTGTCGTGGA. All reactions were incubated in a 96-well plate at 95°C for 5 min, followed by 40 cycles of 95°C for 15 s, 65°C for 15 s, and 72°C for 32 s. Each sample was performed in triplicate for analysis. Finally, at the end of the PCR cycles, melting curves were described to confirm the specificity of the expected PCR products. The relative expression levels of each miRNA were calculated according to the 2−∆∆CT method, as described previously.Citation15 For group-wise comparisons, Student’s t-test (two groups), ANOVA or the Kruskal–Wallis test (n group) was used as appropriate. All statistical tests performed were two-sided. In addition, a significance level of p < 0.05 was considered statistically significant. All statistical analysis was carried out by SPSS (version 13.0) software (Statistical Package for the Social Science, Chicago, IL, USA).
RESULTS
A total of 50 subjects were in the study group, which included 30 ESRD patients and 20 healthy controls. In , the clinical characteristics of the study population are summarized.
Table 1. Baseline clinical data of the subjects.
Expression of miRNAs in Plasma Samples
To date, there is no reliable housekeeping miRNAs in studying circulating miRNAs. Therefore, we supplemented the samples with recombinant cel-lin-4, which can be consistently detected by RT-qPCR, and no significant differences in the raw Ct values of cel-lin-4 were detected among the three groups (p = 0.825, Kruskal–Wallis test); thus cel-lin-4 could be used to normalize the difference in the efficiency of RNA isolation.
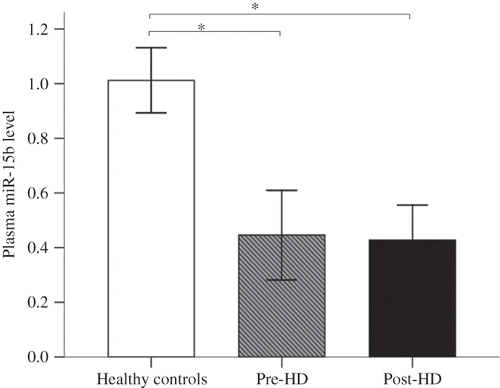
As shown in , the circulating levels of miR-15b significantly differed between patients with ESRD and healthy controls and were significantly down-regulated in prehemodialysis patients compared with healthy controls. Likewise, the circulating levels of miR-15b were also significantly down-regulated in posthemodialysis patients (p < 0.001). However, the circulating levels of miR-15b did not significantly differ between prehemodialysis and posthemodialysis (p > 0.05).
Figure 1. Circulating miRNAs in patients with ESRD and healthy controls.
Notes: Expression of miR-15b in plasma obtained from patients with ESRD (n = 30) and healthy controls (n = 20), as determined by RT-qPCR. Plasma miR-15b level was indicated by 2−ΔΔCT; prehemodialysis (pre-HD) versus healthy controls, p < 0.001; posthemodialysis (post-HD) versus healthy controls, p < 0.001; pre-HD versus post-HD, p > 0.05. *p < 0.001 probability values were calculated by ANOVA test with Tukey posttest.
Target Prediction and Exploratory Analysis
The targets of miR-15b were predicted by TargetScan (http://www.targetscan.org) to further elucidate its potential role in ESRD. We investigated the significant gene ontology (GO) “biological process” classifications that are significantly enriched among these putative miR-15b genes using the DAVID functional annotation tools (http://david.abcc.ncifcrf.gov/) and analyzed the functional annotation for predicted target sets hoping to shed light on the specific function of miR-15b in the pathophysiology of ESRD. Clustering of overrepresented GO classes in predicted targets of miR-15b in ESRD suggested that the most significant GO terms were genes involved in phosphorus metabolic process, phosphate metabolic process, protein amino acid phosphorylation, and phosphorylation, which might be associated with the pathogenesis of ESRD with disturbances in mineral metabolism. This could suggest that aberrantly expressed circulating miR-15b might be involved in hyperphosphatemia in patients with ESRD by modulating genes in the phosphate metabolic process.
Correlations between Circulating miR-15b and Clinical Parameters
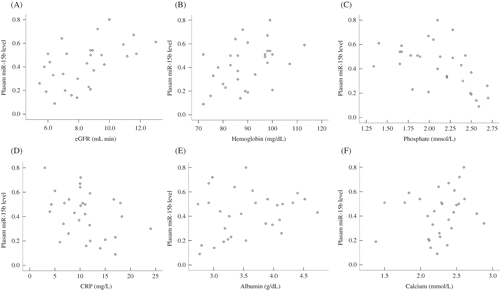
The relative expression levels of circulating miR-15b were calculated by the comparative 2−ΔΔCT. As shown in , the circulating levels of miR-15b positively correlated with both estimated glomerular filtration rate (eGFR; r = 0.502, p = 0.003) and hemoglobin (r = 0.432, p = 0.017) and negatively correlated with phosphate level (r = −0.516, p = 0.004) and CRP (r = −0.377, p = 0.040). However, the circulating levels of miR-15b showed no significant correlation with calcium levels or albumin levels.
DISCUSSION
It has been recognized that miRNAs, as important molecular regulators, can be detected in the circulation and can act as diagnosis and prognosis predictors in diseases like cancer. Moreover, a number of circulating miRNAs can provide important new information for disease. A previous study has demonstrated that the specific circulating miRNAs are reduced in patients with chronic kidney disease (CKD), which has important implication for circulating miRNAs as biomarkers in patients with CKD and for the pathogenesis of uremia.Citation16 In this study, we detected the circulating levels of miR-15b in patients with ESRD receiving maintenance hemodialysis and explored the relationship between circulating miR-15b levels and phosphate metabolism. Compared with healthy controls the circulating miR-15b tends to be significantly reduced in patients with ESRD, and our findings warrant prospective investigation into the role of circulating miRNAs in the pathophysiology and common complications of ESRD.
It is important to realize that hyperphosphatemia, which is closely associated with adverse outcomes in CKD, is an important and modifiable risk factor for death and CVD in patients on hemodialysis.Citation17 Recent studies demonstrated that phosphorus might be involved in the pathophysiological processes of vascular calcification by inducing apoptosis and phenotypic transition to osteoblast or chondrocytes.Citation18 Hyperphosphatemia facilitates the progression of vascular calcification,Citation19 which happens in a large proportion of patients with hemodialysis and is a marker of arterial disease.Citation20 Vascular calcification as a pathogenic factor accelerated the cardiovascular morbidity and mortality in patients with CKD.Citation19 Therefore, disturbance in mineral metabolism appears as the most detrimental factor affecting arteries in CKD patients.Citation20 Although increasing investigations have shown that fibroblast growth factor 23 and Klotho may be involved in hyperphosphatemia,Citation21–23 more importantly, we found the circulating levels of miR-15b were correlated with the phosphate level. This exploratory analysis demonstrated that the variation in circulating levels of miR-15b might affect the critical biological process involved in phosphate metabolisms, an important mechanism that warrants further investigation. The findings suggest that the circulating miR-15b might be involved in the formation and progression of ESRD. However, how miR-15b and phosphate metabolism in patients with ESRD interact with each other in detail is unknown. It is known that the miRNA target prediction algorithm includes false positives and false negatives, and our clustering enrichment analysis is based on predicted genes to be estimated by circulating miR-15b. A clear understanding of how miR-15b regulated its putative targets can only be established with a functional study.
The extracellular miRNAs packed in membrane-bound vesicles that change in numbers, cellular origin, and composition according to the disease state.Citation6,24,25 These vesicles are not only byproducts released from cell apoptosis or activation, but are also involved in the cell–cell mechanism of communication. It is known that phosphate can induce apoptosis of vascular smooth muscle cells (VSMCs),Citation26,27 which leads to production of matrix vesicles that contained the protein BAX (BCL2-associated X protein).Citation27 miR-15b has also been shown to regulate cell proliferation and apoptosis by targeting cell cycle proteins and the antiapoptotic Bcl-2 gene,Citation13,28,29 in spite of no studies done on the expression of miR-15b in VSMCs’ apoptotic body. However, in our study, miR-15b is significantly reduced in the blood in patients with ESRD. It was surprising, because one would expect that vascular cells activation or apoptosis could lead to the release of microparticles and remnants of apoptotic cells, thus increasing the expressed level of circulating miRNAs. Nevertheless, accumulating evidence also suggested that apoptotic bodies and microparticles can be transferred to other cell types.Citation30 Low circulating levels might result from the increased delivery of miR-15b to recipient cells and contribute to disturbance in phosphate metabolism and could be involved in the development of osteoblast-like cells in patients with ESRD.Citation14 Therefore, it is at least theoretically probable that circulating miR-15b is involved in the development of the ESRD. However, it is clear that further investigation is needed to confirm the view.
In addition, we found that circulating miR-15b levels positively correlate with eGFR and hemoglobin, while inversely correlate with phosphate level and CRP. Moreover, our study revealed that the circulating levels of miR-15b did not significantly differ between prehemodialysis and posthemodialysis, indicating that hemodialysis might not lead to the significant changes in the plasma level of miR-15b, and the slight changes of plasma miR-15b might be caused by the responsiveness to hemodialysis. Although preliminary, our finding suggests that miR-15b may be a potential biomarker for monitoring disease.
A few inadequacies of this study should be considered when interpreting the results of this study. First, because of the sample size, we cannot provide enough power to assess the relationships between the change in circulating miR-15b levels and the clinical characteristics with time. Second, other miRNAs, except for miR-15b, might have important predictive values and significant functional roles in the development of ESRD with abnormal phosphate metabolism. In addition, the impact of drug treatment on circulating miR-15b is unknown and requires further study.
In conclusion, this is the first study to survey the relationship between circulating miR-15b levels and phosphate metabolism. The study specifically addressed the level and regulation of miR-15b. However, the circulating levels of miR-15b in patients with ESRD did not significantly differ between prehemodialysis and posthemodialysis. It was also found that there is a correlation between the circulating levels of miR-15b and phosphate levels in patients with ESRD. We still need to confirm these data in large clinical populations and investigate the causal relation between miR-15b and phosphate metabolism. miR-15b, however, deserves special consideration.
ACKNOWLEDGMENT
We sincerely thank all of the patients with ESRD receiving hemodialysis and healthy controls that provided plasma samples.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297.
- Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol. 2009; 4:1255–1266.
- Sequeira Lopez ML, Gomez RA. Novel mechanisms for the control of renin synthesis and release. Curr Hypertens Rep. 2010;12:26–32.
- Li JY, Yong TY, Michael MZ, Gleadle JM. Review: The role of microRNAs in kidney disease. Nephrology (Carlton). 2010;15:599–608.
- Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Comprehensive analysis of microRNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int. 2009;29:749–754.
- Arroyo JD, Chevillet JR, Kroh EM, . Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–5008.
- Zhao H, Shen J, Medico L, Wang D, Ambrosone CB, Liu S. A pilot study of circulating miRNAs as potential biomarkers of early stage breast cancer. PLoS One. 2010;5:e13735.
- Fichtlscherer S, De Rosa S, Fox H, Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684.
- Tijsen AJ, Creemers EE, Moerland PD, MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010;106: 1035–1039.
- Lorenzo Sellares V, Torregrosa V. [Changes in mineral metabolism in stage 3, 4, and 5 chronic kidney disease (not on dialysis)]. Nefrologia. 2008;28(Suppl 3):67–78.
- Goettsch C, Rauner M, Pacyna N, Hempel U, Bornstein SR, Hofbauer LC. miR-125b regulates calcification of vascular smooth muscle cells. Am J Pathol. 2011;179:1594–1600.
- Boucher JM, Peterson SM, Urs S, Zhang C, Liaw L. The miR-143/145 cluster is a novel transcriptional target of Jagged-1/Notch signaling in vascular smooth muscle cells. J Biol Chem. 2011;286:28312–28321.
- Finnerty JR, Wang WX, Hebert SS, Wilfred BR, Mao G, Nelson PT. The miR-15/107 group of microRNA genes: Evolutionary biology, cellular functions, and roles in human diseases. J Mol Biol. 2010;402:491–509.
- Gao J, Yang T, Han J, MicroRNA expression during osteogenic differentiation of human multipotent mesenchymal stromal cells from bone marrow. J Cell Biochem. 2011;112: 1844–1856.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408.
- Neal CS, Michael MZ, Pimlott LK, Yong TY, Li JY, Gleadle JM. Circulating microRNA expression is reduced in chronic kidney disease. Nephrol Dial Transplant. 2011;26:3794–3802.
- Floege J, Kim J, Ireland E, Serum iPTH, calcium and phosphate, and the risk of mortality in a European hemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955.
- Kendrick J, Chonchol M. The role of phosphorus in the development and progression of vascular calcification. Am J Kidney Dis. 2011;58:826–834.
- Ossareh S. Vascular calcification in chronic kidney disease: Mechanisms and clinical implications. Iran J Kidney Dis. 2011;5:285–299.
- Arcidiacono T, Paloschi V, Rainone F, Renal osteodystrophy and vascular calcification. J Endocrinol Invest. 2009; 32:21–26.
- Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): Implications for disorders of phosphate metabolism. Pediatr Nephrol. 2010; 25:591–601.
- Gupta D, Brietzke S, Hayden MR, Kurukulasuriya LR, Sowers JR. Phosphate metabolism in cardiorenal metabolic disease. Cardiorenal Med. 2011;1:261–270.
- Nakai K, Komaba H, Fukagawa M. New insights into the role of fibroblast growth factor 23 in chronic kidney disease. J Nephrol. 2010;23:619–625.
- Tesse A, Martinez MC, Meziani F, Origin and biological significance of shed-membrane microparticles. Endocr Metab Immune Disord Drug Targets. 2006;6:287–294.
- Zampetaki A, Kiechl S, Drozdov I, Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817.
- Valdivielso JM. [Vascular calcification: Types and mechanisms]. Nefrologia. 2011;31:142–147.
- Kockx MM, De Meyer GR, Muhring J, Jacob W, Bult H, Herman AG. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97: 2307–2315.
- Yue J, Tigyi G. Conservation of miR-15a/16-1 and miR-15b/16-2 clusters. Mamm Genome. 2010;21:88–94.
- Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: An essential role for apoptosis. J Hepatol. 2009;50:766–778.
- Prokopi M, Pula G, Mayr U, Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732.