Abstract
We investigated the role of neutrophil gelatinase-associated lipocalin (NGAL) on renal tubular epithelial cell in the renal ischemia/reperfusion injury (IRI) rats. Male Sprague–Dawley rats were randomly assigned to three groups. The control group (n = 5) underwent left nephrectomy. The ischemia/reperfusion (I/R) + normal saline (NS) (n = 5) and I/R + NGAL groups (n = 5) were subjected to 45 min right renal ischemia followed by 48 h of reperfusion after left nephrectomy. The pathological changes of kidney tissues were investigated using hematoxylin–eosin staining; renal tubular epithelial cell apoptosis was detected using terminal dUTP nick-labeling method; expression of apoptosis-regulating protein Fas and Bcl-2 was measured using real-time polymerase chain reaction, Western blot, and immunohistochemical staining. Compared with I/R + NS group, kidney tissues from I/R + NGAL group revealed reduced histological damage and a decreased number of renal tubular epithelial cell apoptosis (9.2 ± 2.53 nuclei or 4.0 ± 0.7 per high-power field vs. 20.3 ± 3.7 nuclei or 8.1 ± 0.3 per high-power field); rats with NGAL showed downregulated fas mRNA (2.34 ± 0.51 vs. 6.84 ± 2.34), fas protein (0.65 ± 0.05 vs. 0.95 ± 0.08), and upregulated bcl-2 protein (0.33 ± 0.05 vs. 0.24 ± 0.03). The results had statistical significance (p < 0.05). We think NGAL could protect against renal IRI and might be related to decreasing tubular epithelial cell apoptosis via adjusting the expression of apoptosis-regulating proteins.
INTRODUCTION
NGAL (neutrophil gelatinase-associated lipocalin, also known as lipocalin-2, siderocalin, uterocalin, and 24p3) belongs to the lipocalin family.Citation1 This ubiquitous 25-kDa protein is secreted by various types of human tissues, including the gastrointestinal tract, the respiratory tract, and the kidneys. In the kidneys, NGAL is synthesized largely in the loop of Henle and collecting ducts with secretion into urine by distal nephrons;Citation2,3 circulating NGAL is freely filtered by glomerulus and uptaken by proximal tubular epithelia via endocytosis.Citation4 Recently, NGAL has emerged as one of the best studied urinary biomarkers of acute kidney injury. The concentration of NGAL increases dramatically in response to tubular injury and precedes rises in serum creatinine by 24 h.2 Meanwhile, many questions with respect to the detailed role of NGAL in renal tubular injury remain unanswered. Mori et al.Citation4 found that a single dose of NGAL (10 μg) dramatically protected the kidney and mitigated azotemia using a mouse model of renal ischemia/reperfusion (I/R) injury (IRI).
Renal IRI is a major cause of acute kidney injury and is commonly seen in clinical practice, usually in renal surgeries, kidney transplantation, acute renal arterial occlusion, and hypovolemic shock. The pathogenesis of IRI is very complex, involving calcium overload, generation of oxygen-free radicals, disorder of mitochondria function, and tubular apoptosis. Tubular apoptosis is considered to be one of the important factors involved in the pathophysiology of IRI.Citation5,6 Apoptosis is an evolutionarily conserved form of cell suicide under the control of numerous genes and is mediated by two signaling pathways: extrinsic or Fas/FasL-mediated cell death and intrinsic or Bcl-2/Bax pathway. Fas is the death receptor, which is initiated by the binding of extracellular ligands, FasL, leading to caspase8 activationCitation7,8 to trigger cell apoptosis. The Bcl-2 family of proteins is composed of B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X (Bax), and so on.Citation9 The ratio of Bcl-2 to Bax determines the susceptibility to apoptosis through regulating the release of cytochrome C from mitochondria.Citation10 Upregulation of the ratio of Bcl-2 to Bax can provide protection against cell apoptosis.Citation11,12
Some evidence illustrated NGAL as a survival factor in cell apoptosis and apoptosis induced in human adenocarcinoma A549 cells by MK886 (a strong proapoptotic agent), or PDK1 (phosphoinositide-dependent kinase 1) inhibitors were accompanied by a dose- and time-dependent increase of NGAL mRNA levels; overexpression of NGAL reduced cell apoptosis by PDK1 inhibitors while downregulation of NGAL expression by small interfering RNA increased the toxicity of a PDK1 inhibitor by approximately 45%.Citation13 Similarly, NGAL protected thyroid carcinoma cells from apoptosis and silencing of NGAL reduced thyroid tumor growth.Citation14 As already stated above, NGAL is endocytosed by proximal tubular epithelia; it can also protect kidney and mitigate azotemia in renal IRI; thus, this caused our interest to investigate the effects of NGAL on renal tubular epithelial cell apoptosis and apoptosis-regulating protein Fas and Bcl-2 in renal IRI rats.
MATERIALS AND METHODS
Renal IRI Model
This study was approved by the Institutional Animal Care and Use Committee, and the procedures were carried out according to National Institute of Health guidelines. Adult healthy male Sprague–Dawley rats (weight 200 ± 20 g) were randomly divided into three groups (n = 5/group). Rats were placed in a heating pad under a warming light to maintain body temperature of 37°C.
Control group
Rats were anesthetized with intraperitoneal pentobarbital (40 mg/kg). The left kidney was removed and then the abdominal cavity was closed in two layers. Normal saline (NS) (0.5 mL) was given at 0.5 h preoperatively and 0.5, 1, and 2 h postoperatively via vein injection.
Ischemia/reperfusion (I/R) group (I/R + NS)
After intraperitoneal anesthesia, the left kidney of the rat was removed and the renal artery and vein of the right kidney were occluded nontraumatically with a clamp. After 45-min ischemia, the clamp was withdrawn. Occlusion was verified visually by a change in the color of the kidneys to a paler shade and reperfusion by a blush. NS (0.5 mL) was given at 0.5 h preoperatively and 0.5, 1, and 2 h postoperatively via vein injection.
NGAL treatment group (I/R + NGAL)
After intraperitoneal anesthesia, the left kidney of the rat was removed and the right kidney was subjected to 45 min of ischemia with atraumatic vascular clamp. NGAL (10 μg) was given at 0.5 h preoperatively and 0.5, 1, and 2 h postoperatively via vein injection. All rats received 10,000 units of penicillin at 0 and 24 h postoperatively, and right kidneys were taken from the body for next stage of experiments 48 h postoperatively.
Histology
Forty-eight hours after reperfusion the right renal tissues were fixed in 10% buffered formalin, and then were embedded in paraffin. Tissues were stained with hematoxylin and eosin for standard histological examination. A pathologist blinded to the groups subjected the tissues to microscopic examination.
Apoptosis of tubular epithelial cells was determined using terminal dUTP nick-labeling (TUNEL) assay. TUNEL staining was viewed with a microscope (Nikon 90i, Nikon Co., Tokyo, Japan). The apoptotic cells were estimated by counting the number of TUNEL-staining nuclei. For each specimen, both positive and negative nuclei from 10 random microscopic fields (×100, ×400) were counted (50 fields/group). Data were expressed as TUNEL-staining cells per microscopic field.
Tubular apoptosis was assessed by counting the number of apoptotic bodies in the most severely injured area after renal IRI, which is the outer medulla of the kidney in all rats.
Immunohistochemistry
After deparaffinization and rehydration, paraffin sections of the kidneys were incubated with 3% hydrogen peroxide for 15 min to quench endogenous peroxidase activity. Tissue sections were placed in a microwave oven for antigen unmasking in 10 mM sodium citrate buffer (pH 6.0). After blocking with 5% goat serum in PBS, tissue sections were incubated with primary rabbit anti-rat polyclonal antibody against Fas (1:100 dilution; Santacruz Co., CA, USA) and Bcl-2 (1:50 dilution; Bioworld Co., Minneapolis, MN, USA) at 4°C overnight. After washing with PBS, sections were incubated with secondary anti-rabbit antibody. Finally, after incubation with H2O2-DAB for 1 min, immunostaining appeared and a blinded observer scored semiquantitatively the quantity of the staining by positive rate (percentage of positive area/microscopic field) via Axioplan 2 imaging KS400 (ZEISS Co., Oberkochen, Germany). For each specimen, two random microscopic fields (×100) were computed (10 fields/group).
Real-Time PCR
Total RNA was isolated from whole kidneys by using TRIzol (Invitrogen Co., Carlsbad, CA, USA). With Promega (Madison, WI, USA) M-MLV1 instructions, 2 μg total RNA was converted to cDNA. Primers for Fas, Bcl-2, and beta-actin are presented in . All polymerase chain reactions (PCRs) were performed in Applied Biosystems 7900HT Fast Real-Time PCR System (Carlsbad, CA, USA) with a final volume of 20 μL and the parameters consisted of 10 min of Taq activation at 95°C, followed by 40 cycles of PCR at 95°C for 15 s, 55°C for 1 min. From each amplification plot, a threshold cycle (Ct) value (defined as the number of PCR cycles where the fluorescence signal exceeded the detection threshold value) and the number of molecules of the genes were calculated. Relative mRNA quantification was done using the equation 2−(ΔCt sample − ΔCt control), where ΔCt equals “Ct sample–Ct beta-actin.”
Table 1. Primer sequence.
Table 2. Positive rates of the apoptotic proteins (mean ± SD; n = 10).
Western Blot
Whole kidneys were pulverized in liquid nitrogen. Small parts of the tissues were suspended in radio-immunoprecipitation assay (RIPA) buffer. After microcentrifugation at 10,700 × g for 30 min at 4°C, supernatant was collected and protein concentration was determined using the bicinchoninic acid (BCA) method. The supernatant containing 40 μg of protein was separated using SDS-PAGE and transferred to a nitrocellulose membrane. Primary antibodies included Fas (C18C12) rabbit mAb (Cell Signaling Technology Inc. (CST), Boston, MA, USA), Bcl-2 (50E3) rabbit mAb (CST), and beta-actin (13E5) rabbit mAb (CST). The membrane was then incubated with an HRP-conjugated anti-rabbit secondary antibody for 1 h. The immunoblotting was visualized using LAS3000 system (Fujifilm Inc., Osaka, Japan).
Statistical Analysis
Data were expressed as mean ± SD. Kruskal–Wallis and Nemenyi tests were used for statistical analysis to compare measurement data among all groups. A p-value of <0.05 was considered significant.
RESULTS
Histopathologic and Tunnel Findings
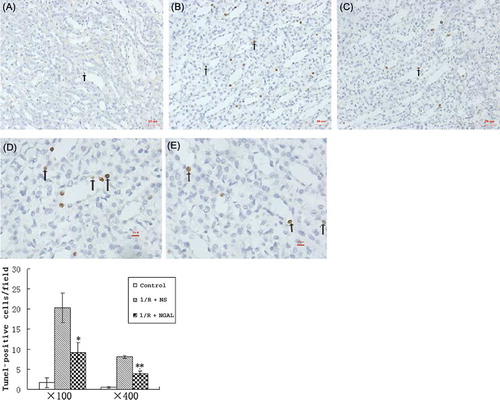
Light microscopy revealed normal kidney morphology in the control group (A) while tubular cell necrosis, hemorrhage, and tubular dilatation were observed in histological specimens from the I/R + NS group (B). These histological alterations were reduced in specimens from the I/R + NGAL group (C). To assess the direct contribution of NGAL to tubular cell apoptosis in IRI, the degree of renal tubular apoptosis was quantified by counting the number of apoptotic bodies using TUNEL assay. Kidneys after IRI showed an amount of TUNEL-positive staining (B and D); in contrast, kidneys subjected to NGAL after IRI had fewer TUNEL-positive cells (C and E) (20.3 ± 3.7 nuclei or 8.1 ± 0.3 per high-power field vs. 9.2 ± 2.53 nuclei or 4.0 ± 0.7 per high-power field, p < 0.05). We also took the approach of systematic random sampling, and similar results were obtained.
Figure 1. Histologic changes in the kidney at the end of 48 h of reperfusion. Tissues were stained with hematoxylin and eosin. Shown are representative histological specimens from the control group (A); renal I/R + NS group (B); and I/R + NGAL group (C). Magnification ×100.
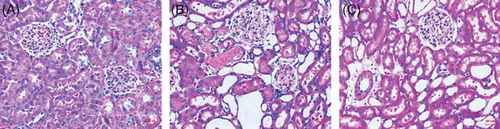
Figure 2. Representative photomicrographs of the outer medulla illustrate apoptotic nuclei. The control group rarely showed apoptotic nuclei (A, magnification ×100); rats subjected to I/R + NS showed TUNEL-positive cells (B, magnification ×100; D, magnification ×400); rats subjected to I/R + NGAL showed a smaller degree of TUNEL-positive cells (C, magnification ×100; E, magnification ×400). Arrows indicate TUNEL-positive cells. *p, **p < 0.05, versus I/R + NS group.
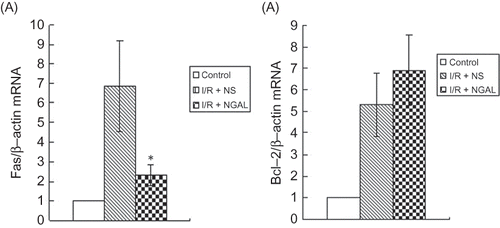
The Effect of NGAL on the Expression of Fas and Bcl-2 mRNA after IRI
To further examine the extent of apoptosis after IRI, real-time PCRs were performed by detecting the endogenous levels of Fas and Bcl-2 genes. The mRNA levels in the control, I/R + NS, and I/R + NGAL groups were determined using real-time PCR and beta-actin was used as a reference gene (). The Fas mRNA level of the I/R + NGAL group was lower than that of the I/R + NS group (6.84 ± 2.34 vs. 2.34 ± 0.51, p < 0.05). The levels of Bcl-2 in the I/R + NS and I/R + NGAL groups were not significantly different (5.30 ± 1.48 vs. 6.91 ± 1.64, p > 0.05).
The Effect of NGAL on the Expression of Fas and Bcl-2 Protein after IRI
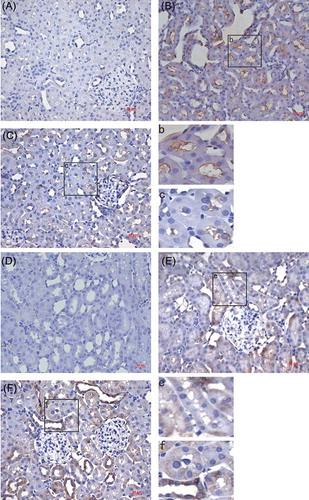
Immunohistochemistry and Western blot were performed by detecting the endogenous levels of Fas and Bcl-2 proteins. Positive staining of Fas and Bcl-2 was observed in both I/R + NS and I/R + NGAL groups and Fas and Bcl-2 immunostaining was only marginal in the control group (, ). Fas staining in I/R + NGAL group was significantly decreased than that in the I/R + NS group (1.76 ± 1.33 vs. 13.7 ± 6.54, p < 0.05) while Bcl-2 staining in I/R + NGAL group was increased than that in the I/R + NS group (18.96 ± 4.98 vs. 4.9 ± 3.38, p < 0.05).
Figure 4. Immunohistochemical staining of Fas in control group (A), I/R + NS group (B), and I/R + NGAL (C); Fas staining was strong on tubular epithelial cell membrane. Bcl-2 in control group (D), I/R + NS group (E), and I/R + NGAL (F); Bcl-2 staining was strong on tubular epithelial cell plasma. Magnification ×100. The magnifications b, c, e, and f were taken from the insert frames.
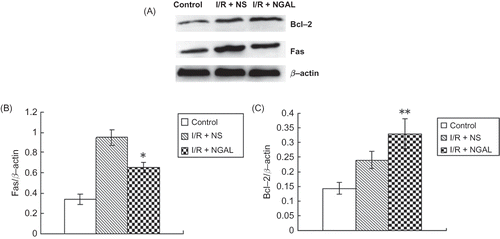
Our Western blot results showed that renal IRI led to a marked increase of Fas and Bcl-2 protein expression in the kidney tissue at 48 h of reperfusion. NGAL treatment in the tissues resulted in a decrease in the protein expression of Fas (, p < 0.05) and an increase of Bcl-2 than that in the I/R + NS group (, p < 0.05).
Figure 5. Immunoblot analysis of Fas and Bcl-2 proteins (A). The density of band was quantified. Values presented are ratios of Fas or Bcl-2 to beta-actin, which was used as an equal protein loading marker (B and C). Results are presented as mean ± SD (n = 5); *p < 0.05, versus IR + NS group; **p < 0.05, versus IR + NS group.
DISCUSSION
This study demonstrated that NGAL provided protection against renal IRI in rats. The main findings are as follows: (1) NGAL efficiently reduced renal tubular damage; (2) NGAL decreased the number of renal tubular epithelial cell apoptosis; (3) NGAL downregulated Fas mRNA and protein expression, and at the same time, upregulated Bcl-2 protein expression.
True extension of IRI is seen at 24–72 h after reperfusion,Citation15–17 so we selected 48 h of reperfusion as the time point to find out the effect of NGAL to the renal IRI. Inducing IRI in rats that underwent nephrectomy and subjecting to 45 min of ischemia to the contralateral kidney followed by 48 h of reperfusion, we observed renal morphological alterations including tubular cell necrosis, hemorrhage, and tubular dilatation. Meanwhile, we found out that tissue damage was ameliorated with NGAL administration. To determine whether NGAL was involved in renal tubular cell apoptosis, we examined renal tissues by TUNEL assay. Apoptotic cells were rare in control tissues; after IRI, the number of apoptotic cells increased to approximately 8.1 ± 0.3 nuclei/high-power field but 4.0 ± 0.7 nuclei/high-power field in NGAL-administrated rats. Mishra et al.Citation18 demonstrated that tubular cells overexpressing NGAL were not TUNEL-positive in mouse models of renal IRI. These findings suggest that NGAL plays an important role in tubular cell apoptosis.
As indicated above, classical Fas/Fasl and Bcl-2/Bax signaling pathways control tubular cell apoptosis. Our results revealed that renal IRI induced significant increase in the expression of Fas and Bcl-2 at mRNA and protein levels. We also found NGAL treatment lowered the levels of Fas mRNA and protein and increased the expression of Bcl-2 protein. Real-time PCR, Western blot, and immunohistochemistry strongly suggested that Fas/Fasl and Bcl-2/Bax signaling pathways might be involved in apoptosis associated with renal IRI and that NGAL might protect against renal IRI by adjusting apoptosis-regulating proteins.
In support of this protective effect is the structure and function of NGAL. Like other lipocalins, NGAL forms a barrel-shaped tertiary structure with a hydrophobic calyx that binds small molecules that are thought to define the biologic activity of the lipocalin family.Citation19 Siderophore, one small iron-binding molecule, is a ligand of this kind.Citation20 NGAL bound to siderophore and iron delivers iron from extracellular environments into intracellular sites. This might remove free and reactive iron, thus stopping reactive iron from producing oxygen radicals that can cause cell apoptosis.Citation2 On the other hand, NGAL releases iron; intracellular iron could be protective and enhance cell repair for a number of reasons. For example, iron is necessary for the R2 subunit of ribonuclease reductase, the enzyme that synthesizes DNA;Citation21 iron can also inhibit apoptosis mediated by p38 MAPK phosphorylation.Citation22 Sun et al.Citation23 used human colon adenocarcinoma cells as a proliferating cell model to validate that desferrioxamine (iron chelator) inhibits cell proliferation and induces apoptosis. With iron deprivation, HL-60 promonocytes bypass differentiation and undergo apoptosis and gene expression of cyclin, Bcl-2, and myc could be inhibited.Citation24 It is indeed possible that both mechanisms are operative in the renal IRI.
In summary, we established a rat renal IRI model and demonstrated that NGAL administration attenuated renal tubular histological injury and inhibited renal tubular epithelial cell apoptosis via Fas/Fasl and Bcl-2/Bax signaling pathways. These findings further support the potential role of NGAL in acute renal injury. Further understanding the mechanism of NGAL involved in acute renal injury can open new targets for diagnostic and therapeutic avenues.
ACKNOWLEDGMENT
This work was supported by research grants from Shanghai PuJiang Foundation (Grant No. 030432).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Flower DR. The lipocalin protein family: Structure and function. Biochem J. 1996;318(1):1–14.
- Schmidt-Ott KM, Mori K, Kalandadze A, . Neutrophil gelatinase associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15(4):442–449.
- Schmidt-Ott KM, Mori K, Li JY, . Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18(2): 407–413.
- Mori K, Lee HT, Rapoport D, . Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115(3):610–621.
- Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17(6):1503–1520.
- Molitoris BA. Transitioning to therapy in ischemic acute renal failure. J Am Soc Nephrol. 2003;14(1):265–267.
- Padanilam BJ. Cell death induced by acute kidney injury: A perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284(4):608–627.
- Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281(5381):1305–1308.
- Yoshida T, Kurella M, Beato F, . Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney Int. 2002;61(5):1646–1654.
- Yang J, Liu X, Bhalla K, . Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science. 1997;275(5302):1129–1132.
- Ye J, Li J, Yu Y, Wei Q, Deng W, Yu L. l-Carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway. Regul Pept. 2010;161(1–3):58–66.
- Wu HH, Hsiao TY, Chien CT. Ischemic conditioning by short periods of reperfusion attenuates renal ischemia/reperfusion. J Biomed Sci. 2009;11:16–19.
- Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391(2):441–448.
- Iannetti A, Pacifico F, Acquaviva R, . The neutrophil gelatinase-associated lipocalin (NGAL), a NFkappaB-regulated gene, is a survival factor for thyroid neoplastic cells. Proc Natl Acad Sci USA. 2008;105(37):14058–14063.
- Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35(3):198–204.
- Ratych RE, Bulkley GB. Free-radical-mediated postischemic reperfusion injury in the kidney. Free Radic Biol Med. 1986;2(5–6):311–319.
- Goujon JM, Hauet T, Menet E, Levillain P, Babin P, Carretier M. Histological evaluation of proximal tubule cell injury in isolated perused pig kidneys exposed to cold ischemia. J Surg Res. 1999;82(2):228–233.
- Mishra J, Ma Q, Prada A, . Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10): 2534–2543.
- Flower DR. Experimentally determined lipocalin structures. Biochim Biophys Acta. 2000;1482(1–2):46–56.
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10(5):1033–1043.
- Hodges YK, Antholine WE, Horwitz LD. Effect on ribonucleotide reductase of novel lipophilic iron chelators: The desferri-exochelins. Biochem Biophys Res Commun. 2004;315(3):595–598.
- Kim BS, Yoon KH, Oh HM, . Involvement of p38 MAP kinase during iron chelator-mediated apoptotic cell death. Cell Immunol 2002;220(2):96–106.
- Sun X, Ge R, Cai Z, Sun H, He QY. Iron depletion decreases proliferation and induces apoptosis in a human colonic adenocarcinoma cell line, Caco2. J Inorg Biochem. 2009;103(7): 1074–1081.
- Alcantara O, Kalidas M, Baltathakis I, Boldt DM. Expression of multiple genes regulating cell cycle and apoptosis in differentiating hematopoietic cells is dependent on iron. Exp Hematol. 2001;29(9):1060–1069.