Abstract
Purpose: To evaluate ocular blood flow dynamics by color Doppler ultrasonography (CDU) in patients with end-stage renal disease (ESRD). Additionally, to investigate the effect of dialysis type on ocular blood flow by comparing the findings of peritoneal dialysis (PD) subjects, hemodialysis (HD) subjects, and healthy controls. Material and methods: Forty patients (21 HD and 19 PD) and 40 controls were included in the study. CDU and spectral analysis of temporal posterior ciliary artery (TPCA) and central retinal artery (CRA) were performed to evaluate peak systolic flow velocity (PSV), end diastolic flow velocity (EDV), and resistive indices (RIs). Ocular blood flows were examined before and after HD. Post-HD findings were compared with those in PD subjects and healthy controls. Results: PSV and EDV values in CRA and TPCA after HD sessions were found to be significantly decreased when compared with pre-dialysis values. There was no statistically significant difference between the pre-dialysis and post-dialysis RI values of both arteries. Systolic and diastolic blood flows in CRA and TPCA were higher and RI values were lower in PD subjects than in HD and controls. No significant difference was seen between HD subjects and controls. Conclusion: After a single HD session, ocular blood flows of patients with ESRD were normalized. But PD subjects show higher systolic and diastolic ocular blood flows than healthy controls, suggesting that HD is more effective than PD for achieving normal ocular blood flow.
INTRODUCTION
End-stage renal disease (ESRD) is associated with ocular abnormalities, which are related to a combination of the uremic state, underlying disorders like diabetes mellitus, and the effects due to dialysis itself. Conjunctival calcifications, osmotic cataracts, ischemic or uremic optic neuropathy, retinal vein occlusion, and morphological changes in the retina and optic disk are all well-known ocular complications of chronic renal failure and dialysis treatment.Citation1–3 These patients may experience visual problems such as decreased visual activity and sudden blindness during dialysis. These feared complications have resulted in increased requirements for examination of the orbital circulation to prevent the disorders that disturb ocular hemodynamics and visual impairment in patients with ESRD.
The treatment of ESRD relies heavily on dialysis (and ultimately, the only definitive treatment is renal transplant). Since there is a shortage of donor organs and a relative shortage of hemodialysis (HD) facilities, the introduction of peritoneal dialysis (PD) has enabled more patients to survive.
Color Doppler ultrasonography (CDU) is a noninvasive and widely used imaging technique which provides selective information on ocular hemodynamics. Measuring ocular blood flows by CDU has been the subject of many studies searching its relation with different pathologies.Citation4–8 However, the effect of HD on retrobulbar vascular hemodynamics has received limited attention in the literature. One study found that retinal circulation was affected by HD and associated changes in systemic circulatory parameters such as systemic blood pressure, hematocrit, and the amount of fluid removed by using a laser Doppler velocimetry.Citation9 Another study demonstrated that choroidal and retinal blood flows were higher before HD.Citation10 In addition, retrobulbar circulation has been found to be disturbed after a single HD session.Citation11 However, to the best of our knowledge, the effect of PD on retrobulbar blood flow by using CDU in patients with ESRD has never been described in previous studies. The aim of this study was to evaluate choroidal and retinal vascular flow dynamics by CDU in patients with ESRD and to investigate the effects of dialysis type on ocular blood flow by comparing the findings of PD subjects, HD subjects, and healthy controls.
MATERIALS AND METHODS
Following approval from the institutional review board and written informed consent from patients and healthy subjects, this study enrolled a total of 80 subjects. Twenty-one patients (9 females and 12 males) aged between 21 and 82 years (mean 50 ± 18 years) undergoing HD treatment; 19 patients (12 females and 7 males) aged between 22 and 75 years (mean 41.3 ± 14.5 years) undergoing PD treatment; and 40 healthy individuals (9 males and 31 females) aged between 23 and 81 years (mean 43.1 ± 14.5 years) were prospectively evaluated.
At the time of the study, the HD patients were undergoing three dialysis sessions per week and for 5–110 months. All HD patients were receiving standard treatment protocols and had dialysis dose (Kt/V value > 1.2). PD patients had been on regular continuous ambulatory peritoneal dialysis (CAPD) modality treatment for at least 1 year, had good compliance to the technique, and had remained in the same CAPD modality in the last year of therapy. Patients on CAPD were receiving four exchanges per day using standard dialysis bags (6–8 L/day).
The causes of renal failure in HD group were pyelonephritis (n = 1), hypertensive nephrosclerosis (n = 12), polycystic kidney disease (n = 2), calculus (n = 2), nephrotic syndrome (n = 1), obstructive nephropathy (n = 1), and amyloidosis (n = 2). The causes of renal failure in PD group were hypertensive nephrosclerosis (n = 11), polycystic kidney disease (n = 1), amyloidosis (n = 1), glomerulonephritis (n = 6), and renal vascular disease (n = 1). Patients with diabetes mellitus, congestive heart failure, and stroke were not included in the study. In both groups patients with systemic hypertension had been under control by antihypertensive medications, including angiotensin-converting enzyme inhibitors and calcium channel blockers during the study.
Before performing CDU each subject underwent a general ophthalmologic examination, including intraocular pressure (IOP) measurement. The measurement of IOP was taken on the same day between 8:00 am and 11:00 am by the same ophthalmologist. Mean IOP and systemic arterial blood pressure were measured after a 15-min resting period before and after HD. In HD subject’s ocular blood flow was estimated 30 min before and after HD followed by a 10-min rest interval in the supine position. Both eyes were examined with the exception of two patients (who had one eye enucleated).
The same radiologist experienced in Doppler sonography for 4 years performed all measurements by using color Doppler on a Sonoline Antares sonographic system (Siemens AG, Munich, Germany) with a VF 13-5 MHz transducer. Ultrasound gel was gently applied to the external surface of the upper eyelid and care was taken to avoid applying any pressure to the orbit. The central retinal artery (CRA) and temporal posterior ciliary artery (TPCA) were examined in each subject. All measurements were done with an angle-correction cursor that was adjusted parallel to the direction of blood flow (with a limit of 60°).
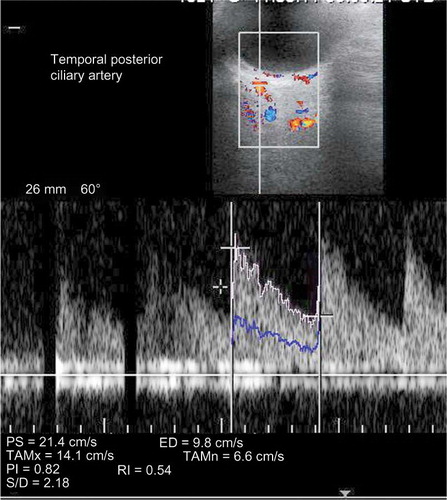
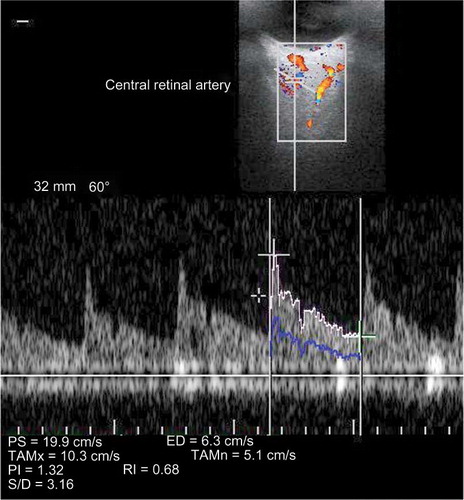
The TPCA was visualized immediately posterior to the globe and lateral to the optic nerve shadow. The CRA was visualized within the retrolaminar portion of the optic nerve where the vessel maintains a straight course ( and ).
The following data were recorded for each of the above-mentioned arteries: (a) peak systolic flow velocity (PSV) defined as the highest velocity of the blood flow during the systolic phase of three cardiac cycles; (b) end-diastolic flow velocity (EDV) defined as the velocity of blood flow at the end of diastolic phase of the cardiac cycle; and (c) resistive index (RI) defined as (PSV – EDV)/PSV.
Statistical Analysis
Data were analyzed using SPSS software 15.0 programs (SPSS Inc., Chicago, IL, USA). For assessing the change in HD subjects before and after dialysis, t-test was used. The changes from pre to post in PSV, EDV, and RI values measured at two locations (central retinal and posterior temporal) were evaluated in the HD subjects. For comparing post-HD values with those of PD and normal subjects, one-way ANOVA, when a significant result was obtained, a Bonferroni adjustment was done for multiple comparisons, was used to test if mean PSV, EDV, and RI values measured at two locations, central and temporal, differed among HD, peritoneal, and normal subjects.
The results of measurements were expressed as mean ± SD and p < 0.5 was considered as significant.
RESULTS
There were no significant differences between PD patients, HD patients, and the control group with respect to age (p = 0.224).
The systolic and diastolic blood pressure measurements are presented in . There were significant differences for systolic (p = 0.01) and diastolic (p = 0.001) blood pressures among groups.
Table 1. Characteristics of patients and controls.
Ophthalmologic examination revealed similar findings in both groups. In HD group 2 patients showed optic atrophy, 6 patients had corneoconjunctival calcifications, and 5 patients showed cataract, whereas in PD group 2 patients showed optic atrophy, 3 patients showed conjunctival degeneration, and 4 patients had cataract at their ophthalmologic examination.
The IOP values are also described in and are within normal limits in all three groups (p = 0.216). The mean PSV values in CRA and TPCA decreased significantly following HD (p < 0.001 and p = 0.003, respectively). The mean EDV values in CRA and TPCA after HD session were found to be significantly decreased when compared with pre-dialysis session (p = 0.022 and p = 0.001, respectively). There was no statistically significant difference between the pre-dialysis and post-dialysis RI values in CRA (p = 0.643) and TPCA (p = 0.274) ().
Table 2. Mean flow values of the retrobulbar vessels of HD subjects both before and after HD.
Table 3. Mean flow values of ocular vessels of PD subjects, HD subjects, and normals.
The mean PSV values for CRA and TPCA were significantly higher for PD patients compared with HD (in both arteries p < 0.0001) and normal subjects (in both arteries p < 0.0001). The mean EDV values for CRA and TPCA were significantly higher for PD patients compared with HD (in both arteries p < 0.0001) and normal subjects (in both arteries p < 0.0001). There was no statistically significant difference between the HD and normal subjects’ PSV and EDV values in either CRA (p = 0.173 and p = 0.366, respectively) or TPCA (p = 0.802 and p = 0.749, respectively).
The mean RI values for CRA and TPCA were significantly lower for the PD group compared with HD patients (p = 0.001 and p < 0.0001, respectively) and normal subjects (p = 0.001 and p = 0.012, respectively).
There was no statistically significant difference between the HD and normal subjects’ RI values in CRA (p = 0.864) and TPCA (p = 0.115). The retrobulbar blood flow velocities and resistivity indices of the subjects are presented in .
DISCUSSION
The posterior ciliary artery provides a blood supply to the optic nerve head and choroidal layer, whereas the CRA provides a blood supply to the retina. Therefore, measurement of the blood flow velocities of PCA and CRA may be good indicators of the capillary blood flow of these vital structures.Citation12
HD and PD are used for treatment of volume overload and uremic symptoms in patients with ESRD. Fluids and dissolved substances (electrolytes, urea, glucose, albumin, and other small molecules) are exchanged from the blood. End-stage renal failure in combination with any type of dialysis may have complications due to either excessive loss of fluid, which may result in hypovolemic shock or hypotension, or excessive fluid retention which can result in hypertension and edema.
In our study, we found that systemic blood pressure and ocular blood flow decreased significantly after a HD session, which suggests that the systemic loss of intravascular volume is directly responsible for the decrease in the systolic and diastolic flow velocities of the retrobulbar vessels.
Ocular blood flow is affected by systemic blood pressure. This was emphasized by several authors. Polak et al.Citation13 found a positive correlation between orbital blood flow and systemic blood pressure. Similarly, Williamson et al.Citation14 demonstrated changes in orbital blood flow velocities according to age, systemic blood pressure, smoking, and blood viscosity and found a significant positive correlation between PSV in ophthalmic artery (OA) and CRA and systolic blood pressure.
We found that systolic and diastolic blood flow velocities of TPCA and CRA decreased, whereas RI values of TPCA and CRA vessels remained the same after HD. This confirms a previous study by Tosun et al.,Citation11 but they did not have any controls within their study. Saygili et al.Citation10 revealed systolic and diastolic blood flow velocities of CRA, OA, and TPCA before a HD session were higher and RI values for these vessels were lower in patients with ESRD than controls. But they did not check whether they normalized following HD.
In this study, we demonstrate that IOP decreased significantly after a HD session. Intradialytic alterations in IOP have been reported in previous studies.Citation15 IOP was reported to be decreased or increased or remained constant during dialysis.Citation16,17 Alterations in IOP can also affect ocular hemodynamics. By the decrease in plasma volume during HD, there is a relative increase in the concentration of plasma protein that increases the plasma colloid osmotic pressure with subsequent transfer of water from the aqueous humor into the plasma causing a reduction in IOP.Citation15
We also found systolic and diastolic blood flow velocities of TPCA and CRA were higher before a HD session, but after HD they showed no significant difference compared with normals. This suggests that dialysis itself does not adversely affect retrobulbar blood flow velocities, but rather normalizes. This was also claimed by Niutta et al.Citation18 who described choroidal perfusion defects mainly in the posterior pole and nasal region of choroids by using fluoroangiography in their study with HD patients. They suggested that choroidal changes in HD patients were likely due to early atherosclerosis of the choroidal membrane rather than HD.
To the best of our knowledge, there is no study that evaluated the role of PD in ocular blood flow patterns. In our study, patients undergoing PD showed higher systolic and diastolic blood flow velocities of TPCA and CRA than HD subjects and normals.
This may be the result of extracellular volume overload due to installation of dialysate within the peritoneal cavity, especially after residual renal function declines.Citation19 Mean RI for the PD group was significantly lower compared with the HD group and normals with similar differences in both arteries. Following Poiseuille’s law, the blood flow varies inversely with viscosity, suggesting that increased blood flow leads to decreased viscosity and decreased vascular resistance.Citation20 Decreased RI may also occur in response to vasodilatation, but we did not assess vascular dimensions as part of our investigation.
In conclusion, our results showed a correlation between higher blood pressure and retrobulbar blood flow. In HD subjects, higher ESV and PSV flows normalize after a HD session, suggesting that HD is more effective in controlling systemic blood pressure and also ocular blood flow.
Conversely, PD subjects showed higher ocular blood flows and lower RIs than post-HD subjects and normals, suggesting that fluid retention occurs in PD subjects. Ultimately, the clinical importance of these findings needs to be assessed in larger cohort of studies with longer term follow-up.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Hilton AF, Harrison JD, Lamb AM, Petrie JJ, Hardie I. Ocular complications in hemodialysis and renal transplant patients. Aust J Ophthalmol. 1982;10:247–253.
- Stibor V, Lachmanova J, Tomasek R. Changes in the ocular fundus in patients with chronic kidney failure on regular dialysis therapy. Cesk Oftalmol. 1989;4:241–252.
- Evans RD, Rosner M. Ocular abnormalities associated with advanced kidney disease and hemodialysis. Semin Dial. 2005;18:252–257.
- Alp MN, Ozgen A, Can I, Cakar P, Gunalp I. Colour Doppler imaging of the orbital vasculature in Graves’ disease with computed tomographic correlation. Br J Ophthalmol. 2000;84(9):1027–1030.
- Goebel W, Lieb WE, Ho A, Sergott RC, Farhoumand R, Grehn F. Color Doppler imaging: A new technique to assess orbital blood flow in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 1995;36(5):864–870.
- Karakucuk S, Goktas S, Aksu M, . Ocular blood flow in patients with obstructive sleep apnea syndrome (OSAS). Graefes Arch Clin Exp Ophthalmol. 2008;246(1):129–134.
- Iveković R, Lovrencić-Huzjan A, Mandić Z, Talan-Hranilović J. Color Doppler flow imaging of ocular tumors. Croat Med J. 2000;41(1):72–75.
- Karaali K, Senol U, Aydin H, Cevikol C, Apaydin A, Lüleci E. Optic neuritis: Evaluation with orbital Doppler sonography. Radiology. 2003;226(2):355–358.
- Nagaoka T, Takeyama Y, Kanagawa S, Sakagami K, Mori F, Yoshida A. Effect of hemodialysis on retinal circulation in patients with end stage renal disease. Br J Ophthalmol. 2004;88:1026–1029.
- Saygili OB, Pelit A, Torun D, . Value of color Doppler ultrasonography in the evaluation of orbital vascular flow in endstage renal disease patients undergoing hemodialysis. Acta Radiol. 2004;45:854–858.
- Tosun O, Davutluoglu B, Arda K, . Determination of the effect of a single hemodialysis session on retrobulbar blood hemodynamics by color Doppler ultrasonography. Acta Radiol. 2007;48(7):763–767.
- Ahmetoglu A, Erdol H, Simsek A, Gokce M, Dinc H, Gumele HR. Effect of hypertensi on and candersartan on the blood flow velocity of the extraocular vessels in hypertensive patients. Eur J Ultrasound. 2003;16:177–182.
- Polak K, Polska E, Lukscha A, . Choroidal blood flow and arterial blood pressure. Eye. 2003;17:84–88.
- Williamson TH, Lowe GDO, Baxter GM. Influence of age, systemic blood pressure, smoking and blood viscosity on orbital velocities. Br J Ophthalmol. 1992;76:690–691.
- Tokuyama T, Ikeda T, Sato K. Effect of plasma colloid osmotic pressure on intraocular pressure during hemodialysis. Br J Ophthalmol. 1998;82(7):751–753.
- Tawara A, Kobata H, Fujisawa K, Abe T, Ohnishi Y. Mechanism of intraocular pressure elevation during hemodialysis. Curr Eye Res. 1998;17:339–347.
- Austin JN, Klein M, Mishell J, . Intraocular pressures during high-flux hemodialysis. Renal Fail. 1990;12:109–112.
- Niutta A, Spicci D, Barcaroli I. Fluoroangiographic findings in hemodialyzed patients. Ann Ophtalmol. 1993;25:375–380.
- Tzamaloukas AH, Saddler MC, Murata GH, . Symptomatic fluid retention in patients on continuous peritoneal dialysis. J Am Soc Nephrol. 1995;6:198–206.
- Berne R, Levy M. Hemodynamics. In: Berne R, Levy M, eds. Physiology. 4th ed. St. Louis, MO: Mosby; 1998.