Abstract
Both apelin and parathyroid hormone (PTH) are endogenous ligands for G-protein-coupled receptors. Apelin acts as a mitogenic agent for osteoblasts, and metabolic bone abnormalities are frequently seen in hemodialysis (HD) patients because of hyperparathyroidism. The aim of this study was to analyze plasma apelin levels in HD patients and to determine whether they are related to PTH concentrations. A total of 23 HD patients [15 men and 8 women, with a mean (SD) age of 54.2 (4.4) years and a mean body mass index (BMI) of 25.0 (4.1) kg/m2] were studied and compared with 15 healthy subjects [6 men and 9 women, with a mean (SD) age of 51.3 (13.6) years and a BMI of 27.0 (4.3) kg/m2]. Plasma apelin-36 was measured using an enzyme immunometric assay method and PTH was measured by ELISA. There was no significant difference in apelin levels between the patients [0.80 (0.6) ng/mL] and the healthy subjects [0.83 (0.23) ng/mL]. There was a positive correlation between apelin and PTH (r = 0.66, p = 0.0001). The patients with PTH >300 pg/mL had significantly higher plasma apelin levels [1.17 (0.7) ng/mL] compared with the patients with PTH <300 pg/mL [0.50 (0.15) ng/mL] (p = 0.003). In conclusion, HD patients with secondary hyperparathyroidism have high plasma apelin levels, which suggest that apelin may protect bone in HD patients by acting as an osteoblastic factor.
INTRODUCTION
Tatemoto et al.Citation1 isolated a 36-amino-acid peptide, which was named apelin (from APJ endogenous ligand). Boucher et al.Citation2 identified apelin mRNA in adipocytes from murine and human subcutaneous adipose tissue. Apelin production is upregulated in some models of obesity. It seems to exert predominantly “beneficial” effects on cardiovascular physiology and insulin sensitivity. Apelin seems to reduce blood pressure and have inotropic activity. Changes in plasma apelin levels have been described in some diseases, with conflicting results.Citation3
Recent studies have shown that human osteoblasts express apelin and APJ and that apelin exerts a beneficial effect on bone metabolism by stimulating human osteoblast proliferation.Citation4 In mice, apelin enhances osteoblastic MC3T3-E1 cell proliferation and stimulates anti-apoptotic mechanisms.Citation5
Alterations in bone metabolism are present in chronic kidney disease (CKD) patients, particularly in hemodialysis (HD) patients. These patients present with several conditions, such as phosphate retention, hypocalcemia, and calcitriol deficiency, which in turn stimulate parathyroid hormone (PTH) secretion and lead to secondary hyperparathyroidism. Persistent hyperparathyroidism has a significant impact on kidney-related bone abnormalities.Citation6
The actions of PTH and apelin are mediated by different G-protein-coupled receptors with theoretically opposite effects, suggesting a potential link between these peptides. There have been no studies addressing the relationship between PTH and apelin. The aim of this study was to describe the plasma apelin-36 concentrations in HD patients and their relationship with PTH and bone mineral density (BMD).
SUBJECTS AND METHODS
Subjects
A total of 23 HD patients [15 men and 8 women, with a mean (SD) age of 54.2 (14.4) years and a mean time on dialysis of 40 (38) months] were recruited from the RenalCor Clinic, Rio de Janeiro, Brazil. The inclusion criteria were age >18 years and patients on maintenance dialysis for at least 6 months. The exclusion criteria were patients with inflammatory diseases, known malignancies, and cardiovascular complications (including uncontrolled hypertension). The dialysis sessions were 3.0–4.5 h three times per week, with a blood flow >250 mL/min, a dialysate flow 500 mL/min, and a bicarbonate buffer. The etiologies of their renal failure were nephroangiosclerosis (N = 8), diabetic nephropathy (N = 5), chronic glomerulonephritis (N = 3), and others (N = 8). The study protocol was approved by the Ethics Committee of the Faculty of Medicine at the Fluminense Federal University. Plasma apelin and PTH levels were also determined in a group of 15 healthy subjects who were similar to patients in gender, age, and body mass index (BMI).
Methods
Nutritional Assessment
The anthropometric measurements were performed using standard techniques immediately after the HD session by a trained staff member (V.O. Leal). The waist circumference (WC), triceps skinfold (TSF), and arm muscle area (AMA) measurements were obtained. The nondominant arm was used for these measurements, except in cases with arterial venous fistula. The TSF was measured using a Lange Skinfold Caliper (Cambridge Scientific Products, Cambridge, MA, USA). The AMA was calculated according to the following formula: AMA = {[MC (cm) – π × TSF (mm)/102/4π]} – n, where n = 10 for males and 6.5 for females and MC refers to the mid-arm circumference. The corresponding percentiles were determined based on the tables developed by Frisancho,Citation7 and the values between 15th and 95th percentiles defined normality.
Dual-energy X-ray absorptiometry (DEXA) examinations were performed using a Prodigy Advance Plus LUNAR (GE Lunar, Madison, WI, USA) densitometer to assess BMD.
Biochemical Variables
Serum albumin, glucose, urea, and creatinine levels were measured using standard laboratory methods. The blood samples were obtained from the arterial HD line before the start of the session, which was after a 10-h overnight fast. The serum was immediately frozen at –80°C for further analysis. Intact PTH was measured by ELISA and plasma apelin-36 was measured by a microplate enzyme immunometric assay kit (Phoenix Pharmaceuticals Inc., Belmont, CA, USA) following the manufacturer’s instructions using an antibody that had 100% specificity for human apelin and 100% cross-reactivity with apelin-36.
Patients who had elevated plasma levels of PTH (>300 pg/mL) were classified as having hyperparathyroidism, following the National Kidney Foundation Guidelines.Citation8 PTH values <70 pg/mL were considered normal for the healthy subjects and values <300 pg/mL were considered normal for the HD patients.
Statistical Analysis
The results were expressed as mean (SD) or percentage change, as applicable. The Student’s t-test was used to examine the difference between the means and the Kruskal–Wallis test was used for nonparametric data. Spearman’s correlation coefficients were used to examine the relationships between variables. Statistical significance was defined by p < 0.05. The statistical analyses were performed using SPSS 11.0 software.
RESULTS
The clinical and biochemical characteristics of the subjects according to PTH levels are given in . Both groups had similar anthropometric measurements.
Table 1. Patient characteristics and serum parameters according to PTH levels.
Table 2. Apelin levels and bone densities according to PTH level.
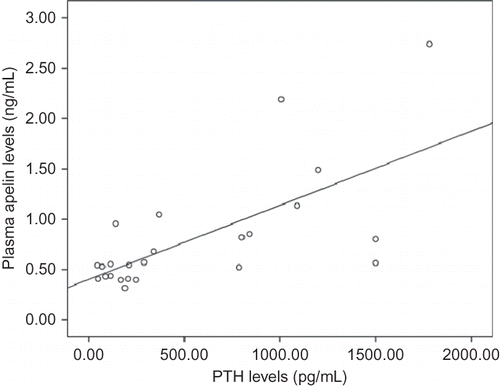
The apelin levels were not significantly different between the HD patients [0.80 (0.6) ng/mL] and the healthy subjects [0.83 (0.23) ng/mL]. The healthy subjects had a mean PTH level of 53.6 (18.5) pg/mL and there was no correlation with the apelin levels. In the HD patients, however, the apelin levels were positively correlated with the PTH levels (r = 0.66, p = 0.0001) () and were not significantly correlated with any other variables.
Figure 2. Plasma apelin levels in HD patients with hyperparathyroidism compared with that in HD patients without hyperparathyroidism (p = 0.003).
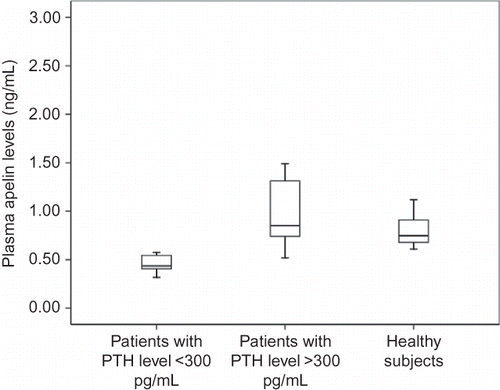
The HD group with higher PTH levels had higher apelin levels [1.17 (0.7) ng/mL] than the group with lower PTH levels [0.50 (0.15) ng/mL] (p = 0.003) ().
There was no significant difference in BMD () between the HD groups with high and low PTH levels.
DISCUSSION
In light of the recently discovered apelin peptide, we have assessed plasma apelin levels in HD patients. The most important finding of the present study was the positive correlation between apelin and PTH levels in HD patients. The apelin levels were higher in the patients with hyperparathyroidism.
The mean apelin concentration in our healthy subjects (0.83 ng/mL) was of the same order as that reported in the literature for apelin-36: 0.84Citation9 and 0.9 ng/mL.Citation10 The apelin levels were not significantly different in the HD patients. The molecular weight of apelin is 4195.9, and it is not a dialyzable molecule.
The literature indicates that plasma apelin is in the normal range for patients on conservative treatments,Citation11 is decreased in HD patients with cardiomyopathy,Citation9 and uremic status was the determinant for decreased plasma apelin regardless of the severity of heart involvement.Citation12 Furthermore, it is increased in patients without cardiovascular disease.Citation13 Our patients were on HD, but they did not have cardiomyopathy.
The positive correlation between apelin and PTH in HD patients may occur because both are mediated by G-protein-coupled receptors that may exert opposite biological functions. In fact, apelin protects human osteoblasts against serum deprivation-induced apoptosis. Apelin binds to the APJ receptor, promotes receptor internalization, inhibits cAMP formation, and activates mitogen-activated protein (MAP) kinase and phosphatidylinositol 3-kinase (PI3 kinase) pathways. By contrast, PTH increases cAMP levels. In addition, apelin can induce osteoblast proliferation through the APJ/PI3 kinase/Akt pathways, and the continuous exposure to PTH inhibits this proliferation.Citation14
Patients with CKD stage 5 have renal osteodystrophy with the associated abnormalities in mineral metabolism. We did not find a correlation between PTH and BMD.
OttCitation15 has examined the results from studies that measure BMD and serum PTH in CKD patients and has observed that none of these studies found a positive relationship between PTH and BMD; either the relationship was not significant or there was a significant inverse correlation. Similarly, we also did not find an association between BMD and apelin.
Apelin is produced by adipocytes and released into the circulation. Its expression in adipose tissue is regulated by nutritional status, and a strong relationship exists between adipocyte-secreted apelin and insulin. A direct action of insulin in the regulation of apelin expression in adipocytes was demonstrated in vivo and in vitro in differentiated adipocytes.Citation2 In the present study, we did not find a correlation between apelin and nutritional status, as assessed by percent body fat, BMI, and WC, and we did not analyze the insulin levels in these patients to determine the other factors that might have released apelin.
The most important limitation of our study is our inability to infer causality from the observed associations. This limitation is inherent in cross-sectional and observational studies.
In conclusion, this study shows normal average apelin-36 levels in HD patients; however, it seems that apelin could play a protective role in bone mainly in patients with hyperparathyroidism who presented high plasma apelin since osteoblasts express apelin and APJ, and that apelin exerts a beneficial effect on bone metabolism by stimulating human osteoblast proliferation. We may speculate that apelin and PTH might be involved in the bone mineral protection in CKD patients.
ACKNOWLEDGMENTS
This work was supported by Coordenação de Aperfeiçoa- mento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Brazil.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Tatemoto K, Hosoya M, Habata Y, . Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476.
- Boucher J, Masri B, Daviaud D, . Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–1771.
- Lago F, Dieguez C, Gómez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18:313–325.
- Xie H, Tang SY, Cui RR, . Apelin and its receptor are expressed in human osteoblasts. Regul Pept. 2006;134:118–125.
- Tang SY, Xie H, Yuan LQ, . Apelin stimulates proliferation and suppresses apoptosis of mouse osteoblastic cell line MC3T3-E1 via JNK and PI3-K/Akt signaling pathways. Peptides. 2007;28:708–718.
- Komaba H, Goto S, Fukagawa M. Critical issues of PTH assays in CKD. Bone. 2009;44:666–670.
- Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981; 34:2540–2545.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl. 3):S1–S201.
- Malyszko J, Malyszko JS, Kozminski P, Mysliwiec M. Apelin and cardiac function in hemodialyzed patients: Possible relations? Am J Nephrol. 2006;26:121–126.
- Foldes G, Horkay F, Szokodi I, . Circulating and cardiac levels of apelin, the novel ligand of the orphan receptor APJ, in patients with heart failure. Biochem Biophys Res Commun. 2003;308:480–485.
- Malyszko J, Malyszko JS, Pawlak K, Mysliwiec M. Visfatin and apelin, new adipocytokines, and their relation to endothelial function in patients with chronic renal failure. Adv Med Sci. 2008;53:32–36.
- Codognotto M, Piccoli A, Zaninotto M. Evidence for decreased circulating apelin beyond heart involvement in uremic cardiomyopathy. Am J Nephrol. 2007;27:1–6.
- Zhang DL, Liao H, Wei YY. Elevation of serum apelin-13 is positively correlated with ADMA in patients on maintenance hemodialysis. Clin Nephrol. 2009;71:405–412.
- Xie H, Yuan LQ, Luo XH. Apelin suppresses apoptosis of human osteoblasts. Apoptosis. 2007;12:247–254.
- Ott SM. Review article: Bone density in patients with chronic kidney disease stages 4–5. Nephrology. 2009;14:395–403.