Abstract
The adequate rituximab (RTX) dosage in ABO-incompatible transplantation (ABO-IKT) remains undetermined. We used two kinds of RTX dosage groups [low RTX (100 mg/m2) and typical RTX (375 mg/m2) dosage groups] according to immunologic risks and investigated the change of B-cell, anti-ABO antibodies, and the clinical outcome in ABO-IKT according to the RTX dose. Fifteen patients with high immunologic risk [panel reactive antibody (PRA) > 50%, retransplant, AB to O transplant] were assigned to typical RTX group and 17 patients without risk were assigned to low RTX group. We compared the changes of B-cell, anti-ABO antibody titer, required number of plasmapheresis (PP), and the clinical outcome after transplantation between the two groups. After infusion of RTX, peripheral blood B-cell counts were successfully depleted to <1% in both groups. Before kidney transplantation (KT), the minimal number of PP to achieve the target titer (1:16) (2.6 ± 2.7 vs. 2.2 ± 2.5; p = 0.66) and the titer reduction rate of anti-ABO antibodies did not differ between the two groups (low RTX: 1.52 ± 1.21 vs. typical RTX: 1.53 ± 1.20, p = 0.94). After KT, anti-ABO antibody titer was suppressed less than 1:32 in both groups up to posttransplant 1 year. The allograft function and infectious complication did not differ between the two groups as well. In ABO-IKT, low RTX is comparable with typical RTX dosing with respect to B-cell depletion, antibody rebound suppression, the effect on clinical outcome in patients with low immunologic risk.
INTRODUCTION
Recent advancements in immune suppression and desensitization protocols have made ABO-incompatible kidney transplantations (ABO-IKTs) feasible, and it provides a greater opportunity for end-stage renal disease patients to undergo transplantation.Citation1 In addition, previous studies have reported similar allograft and patient outcomes between ABO-IKT and IKT.Citation1,2
Most ABO-IKT desensitization protocols are based on rituximab (RTX) and plasmapheresis/intravenous immunoglobulin (PP/IVIG) therapy.Citation2–5 The application of RTX induction therapy in particular has made ABO-IKT feasible without splenectomy with superior allograft outcomes when compared with ABO-IKT with splenectomy.Citation6 However, several questions remain unanswered regarding the use of RTX in ABO-IKT. Specific RTX dosing regimens have not been established and therefore most centers use a dose of 375 mg/m2 as this is the dose used for B-cell lymphoma and autoimmune diseases.Citation2,3
In our preliminary report, we compared the outcomes of ABO-IKT according to the baseline anti-ABO antibody titer.Citation7 In this report, we investigated the feasibility of lowering RTX dosing by evaluating the change of B-cell, anti-ABO antibody titer, and the required number of PP/IVIG compared with those observed in the typical RTX dosage regimens.
SUBJECTS AND METHODS
Patients and Methods
Forty-one cases underwent ABO-IKT at St. Mary’s Hospital, Seoul, from April 2009 to February 2012. Of these patients, nine were excluded [eight patients with high baseline anti-ABO antibody (equal or more than 1:512) and one patient who underwent a significantly modified protocol due to the presence of strong donor-specific anti-HLA antibody]. Thus, 32 patients were enrolled in this study.
Our center’s recent protocol for ABO-IKT has been described previously.Citation7 Typically, we use RTX (MabThera™, Genentech, Inc., San Francisco, CA, USA) at 30 days before transplantation at a dose of 100 mg/m2. However, we use a dose of 375 mg/m2 in highly sensitized patients [panel reactive antibody (PRA) > 50%], previous transplant history, and those who have undergone an AB-to-O type transplantation. Finally, 17 patients were assigned to the low RTX group (100 mg/m2) and 15 patients were assigned to the typical RTX group (375 mg/m2).
PP/IVIG was performed according to the baseline titer in reference to previously recommended guidelines using a COBE Spectra (Gambro BCT, Lakewood, CO, USA).Citation8 The target titer at transplantation was 1:16, but 1:32 was also acceptable when titer levels did not achieve 1:16. Peripheral CD19 and CD20 cell counts were measured by flow cytometry before RTX infusion and just prior to PP/IVIG initiation. When the antibody titer did not achieve the target titer (1:32), an additional PP/IVIG was performed. Posttransplant PP/IVIG was performed only when the titer increased to equal or more than 1:32 within 2 weeks posttransplant. We initiated immunosuppressant treatment comprising tacrolimus, mycophenolate mofetil, and steroids 7 days prior to transplantation. Induction therapy using basiliximab (20 mg) was administered on the day of the surgery and on postoperative day 4.
We compared B-cell counts and antibody titer changes before and after the infusion of RTX between the low RTX and typical RTX dosage groups. To compare antibody titer rebound, we investigated the minimal number of PP/IVIG treatments required to achieve the target titer (1:16) and calculated the titer reduction rate (TRR) based on the following equation:
In addition, we compared clinical outcomes such as infectious complications and acute rejection. For clinical outcome comparisons after kidney transplantation (KT), one patient from the typical RTX dosage group, who died 1 week after transplantation due to cardiac problems, was excluded. This study was approved by the Institutional Review Board of St. Mary’s Hospital, Seoul (KC11RCMI0716).
Statistical Analyses
Statistical analyses were performed using SPSS software (version 15.0; SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation (SD) or counts and percentages, depending on data type. For continuous variables, means were compared using the Student’s t-test. For categorized variables, Pearson’s χ2 test and Fisher’s exact test were used. All tests were two-tailed, and the results were considered significant at p < 0.05.
RESULTS
Comparison of Clinical and Immunologic Characteristics
The comparison of baseline characteristics between the low RTX and the typical RTX dosage groups is presented in . The median titer of baseline anti-ABO antibodies was 1:32 (range, 8–256) in both the low RTX and the typical RTX dosage groups. No significant differences were found in the clinical characteristics, such as age, gender, donor type, follow-up duration, and primary renal disease, in both groups. However, both the proportion of retransplant [0 vs. 9/15 (60%)] and highly sensitized patients [0 vs. 6/15 (40%)] were higher in the typical RTX dosage group. In the typical RTX dosage group, flow cytometric T-cell cross-matching indicated positive results in two patients, and four patients underwent AB-to-O type transplantations.
Table 1. Baseline characteristics of the patient populations.
Table 2. Comparison of acute rejection and infectious complication.
Comparison of the Changes in Peripheral Blood B-Cell Count and Antibody Titer after RTX Infusion
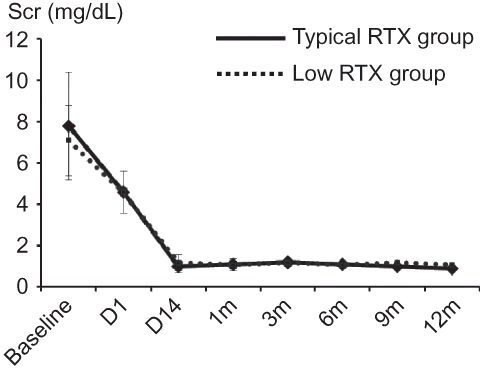
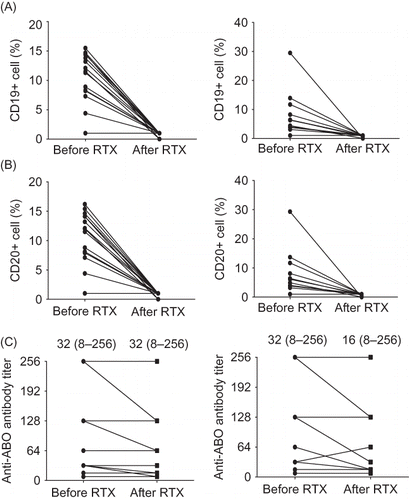
Before RTX infusion, peripheral blood B-cell (CD19+ and CD20+) counts did not differ significantly between the two groups (CD19+: low dose, 9.2% ± 5.0%, and typical dose, 8.2% ± 7.5%, p = 0.67; CD20+: low dose, 9.3% ± 5.2%, and typical dose, 8.1% ± 7.4%, p = 0.62). After RTX infusion, CD19+ and CD20+ cell counts were successfully depleted to <0.1% in both groups. After RTX infusion and before initiating PP/IVIG, antibody titer showed a decreasing pattern in both low RTX and typical RTX dosage groups (p = 0.04) ().
Figure 1. (A) Change of CD19, (B) CD20 positive cell counts, and (C) antibody titer before and after RTX infusion. In low RTX group (left) and typical RTX group (right), CD19+/CD20+ cell counts were successfully depleted to <1%. Antibody titer showed decreasing pattern after the infusion of RTX. RTX, rituximab.
Comparison of the Number of PP/IVIG and the Change in Antibody Titer
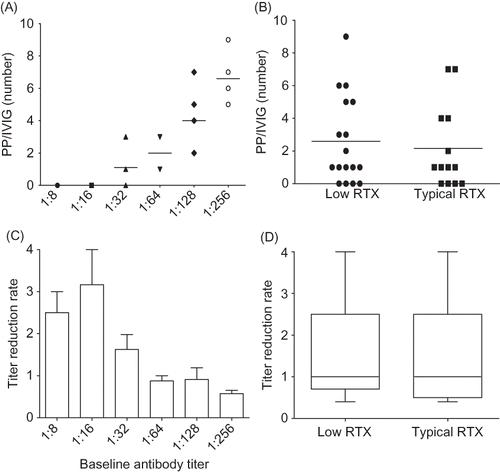
We compared the number of performed PP/IVIG treatments as well as the minimal number of PP/IVIG treatments required to achieve the target titer between the two groups. The performed number of PP/IVIG treatments showed a significant correlation with the baseline antibody titer (r2 = 0.73, p < 0.01) and did not differ significantly between the low RTX and the typical RTX dosage groups (5.3 ± 1.9 vs. 5.6 ± 1.7, respectively, p = 0.66). Minimal number of PP/IVIG treatments showed a significant correlation with baseline antibody titer (r2 = 0.78, p < 0.01), but did not differ between the two groups (low RTX: 2.6 ± 2.7 vs. typical RTX: 2.2 ± 2.5, p = 0.66) (A and B). In both groups, the minimal number of PP/IVG treatments was significantly lower than the performed number of PP/IVIG treatments (p < 0.01). TRR showed a negative relationship with baseline antibody titer (r2 = −0.23, p < 0.01), but TRR in the low RTX and the typical RTX dosage groups did not differ (1.52 ± 1.21 vs. 1.53 ± 1.20, respectively, p = 0.94) (C and D). One patient from each group required posttransplant PP/IVIG treatment.
Figure 2. (A) The minimally required number of PP/IVIG according to baseline anti-ABO antibody titer. Note that the PP/IVIG number significantly increased with the increase of baseline titer (r2 = 0.78, p < 0.01). (B) The minimally required number of PP/IVIG did not differ between low RTX and typical RTX groups. (C) TRR according to baseline antibody titer. It showed significantly decreasing pattern with the increase of baseline antibody titer (r2 = 0.23, p < 0.01). (D) TRR did not differ between low RTX and typical RTX groups. PP/IVIG, plasmapheresis/intravenous immunoglobulin; RTX, rituximab; TRR, titer reduction rate.
Comparison of Changes in Antibody Titer after Transplantation
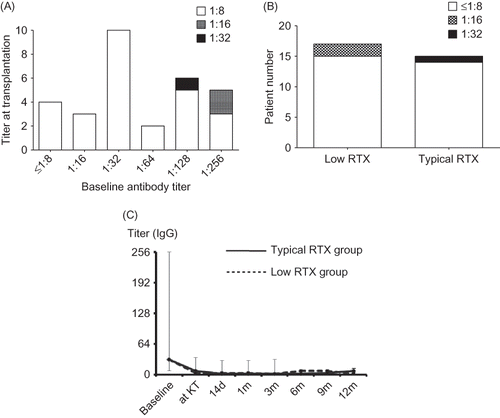
The titer at transplantation was 1:6 (range, 0–16) in the low RTX group and 1:6 (range, 0–32) in the typical RTX dosage group. One patient in the typical RTX dosage group, whose baseline titer was 1:128, received the transplantation at a titer of 1:32 (A and B). In both the low RTX and the typical RTX dosage groups, the antibody titer remained suppressed within a titer level of 1:32 up to 1 year after transplantation (C). Only one patient in the low RTX group showed antibody rebound equal or more than 1:64; however, this value decreased immediately without additional therapy.
Figure 3. (A) The titer at transplantation according to baseline anti-ABO antibody titer. In patients with baseline titer equal or less than 1:1256, most patients took KT at titer of less than 1:16. (B) The distribution of target titer did not differ significantly between low RTX and typical RTX group. (C) In comparison of the change of anti-ABO antibody (IgG) titer, it was suppressed well in both low RTX and typical RTX groups up to 1 year after KT. KT, kidney transplantation; RTX, rituximab.
Comparison of Protocol Biopsy Findings and Clinical Outcome after Transplantation
Allograft function did not differ between the low RTX and the typical RTX dosage groups up to 1 year from KT (). Protocol biopsy was done in 10 patients at 3 months from KT (six cases from the low RTX group and four cases from the usual RTX group) who showed stable allograft function after KT and agreed with this procedure. In all cases, morphologic changes that suggest acute rejection were not detected. C4d stain was diffusely positive in three cases (50%) in the low titer group, and three out of four cases (75%) from the usual RTX group. A total of five cases of biopsy-proven acute rejection were diagnosed in four patients, which did not differ between the two groups. In addition, the development of infectious complications and postoperative bleeding did not differ significantly between the two groups (p = 0.53) ().
DISCUSSIONS
In this study, we investigated the effect of RTX dose on the depletion of peripheral blood B-cells and changes in antibody titer before and after KT. Our results show that both low-dose RTX and the typical RTX dosage groups were capable of suppressing B-cell and antibody titer rebound. Therefore, the treatment dose did not affect the number of PP/IVIG treatments required to achieve the target titer.
First, we determined the dose of RTX in the low RTX group. We chose 100 mg/m2 for low-dose RTX based on previous reports that a dose of 200 mg/body is effective for the prevention of rejection and antibody suppression, and RTX dose as low as 50 mg/m2 completely depletes splenic and peripheral blood B-cells.Citation9–11 Therefore, we thought that RTX dose (100 mg/m2) is effective in preventing acute rejection in patients with immunologically low-risk group.
Second, we evaluated the effect of RTX on peripheral blood B-cell counts, which is known to be rapid— eliminating the cells within a few days—and long term.Citation12 In this study, peripheral B-cell counts were successfully depleted to <1% in all patients, irrespective of RTX dosage. In addition, antibody titer showed a significant decreasing pattern after RTX infusion within a few days before PP/IVIG initiation. This suggests that B-cell depletion by RTX directly affects the antibody producing potential within a few days, and this effect did not differ between the two groups.
Third, we compared antibody titer rebound between the two groups by investigating the minimal number of PP/IVIG treatments required to achieve the target titer and TRR; the minimal number of PP/IVIG was significantly associated with the baseline antibody titer, but not with RTX dose. The calculated TRR showed a significant decreasing tendency with an increase in baseline antibody titer, which—consistent with a previous study—suggests a higher antibody rebound titer after PP/IVIG in those patients.Citation13 However, TRR did not increase in the low RTX group compared with the typical RTX dosage group. The above findings suggest a similar suppressive effect on antibody production between the low RTX and the typical RTX dosage groups.
The effect of B-cell depletion by RTX treatment was sustained for almost 1 year.Citation9 Hence, usually antibody titers should be suppressed until B-cell recovery. When antibody titer rebounded up to a titer equal or more than 1:64, it was associated with AMR development.Citation14 In this study, antibody titer was suppressed within 1:32 until the last follow-up in most patients from both groups. Only one patient in the low RTX dosage group showed antibody rebound to a titer of 1:64 at 6 months after KT, but the antibody titer decreased immediately, and AMR did not occur. This suggests a similar long-lasting RTX effect in both groups.
Stronger immune suppression can increase the risk for infectious complications. Previous reports showed increased viral infection in ABO-IKT with RTX and PP treatments.Citation15–17 In both groups of this study, the performed number of pretransplant PP/IVIG treatments and immune suppressant regimens did not differ, but the RTX dose was different. In contrast to our expectation, low RTX dosage did not decrease opportunistic infections compared with the typical RTX dose. However, long-term RTX complications remain unknown. The comparison of long-term adverse effects, such as late-onset opportunistic infection, requires further investigation.
After transplantation, all patients showed immediate recovery of graft function and the allograft function during posttransplant 1 year were favorable in both groups. The rate of acute rejection was around 10% in both groups and it is comparable with ABO-compatible KT in our center.Citation18 In protocol biopsies, no subclinical rejection was detected in either group. Diffused C4d staining that was proposed as a marker for accommodation and less severe immune reaction in ABO-IKT did not differ between two groups as well.Citation19–21
A limitation of this study includes the fact that we did not use low-dose RTX in patients with immunologic risk factors, such as high PRA or retransplantation. Therefore, it is unclear whether the similarly favorable outcome in both groups can be attributed to the higher RTX dose in typical RTX group in spite of the additional immunologic risk.
Nevertheless, this is the first report to prove similar PP/IVIG requirements in low-dose RTX protocols compared with typical RTX dosing protocols in ABO-IKT. The required number of PP/IVIG with typical RTX dosing has previously been reported.Citation8,22 In another study that investigated the minimum required number of PP/IVIG treatments, two RTX doses and alemtuzumab for induction therapy were used in some patients, which differs from the widely used ABO-IKT protocol.Citation13 A Japanese group proved the effectiveness of low-dose RTX in ABO-IKT; however, they could not investigate its effect on the required number of PP/IVIG treatments because they performed a fixed number of double-filtration PP treatments.Citation11
In summary, low-dose RTX can successfully deplete B-cells and suppress anti-ABO antibodies, hence did not increase the required number of PP/IVIG compared with typical RTX dose. After KT, anti-ABO antibody remained suppressed up to 1 year from KT, and clinical outcome was favorable in the low-dose RTX group. The results of this study suggest that the low-dose RTX regimen could be used safely in the cases of ABO-IKT without immunologically high risk.
ACKNOWLEDGMENT
This study was supported by a grant (A092258) from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
REFERENCES
- Ichimaru N, Takahara S. Japan’s experience with living-donor kidney transplantation across ABO barriers. Nature Clinical Practice Nephrology. 2008;4:682–692.
- Genberg H, Kumlien G, Wennberg L, Berg U, Tydn G. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: A 3-year follow-up. Transplantation. 2008;85:1745–1754.
- Sonnenday CJ, Warren DS, Cooper M, . Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004;4:1315–1322.
- Gloor JM, Stegall MD. O incompatible kidney transplantation. Current opinion in nephrology and hypertension. 2007;16:529–534.
- Tyden G, Donauer J, Wadstrom J, . Implementation of a protocol for ABO-incompatible kidney transplantation: A three-center experience with 60 consecutive transplantations. Transplantation. 2007;83:1153–1155.
- Fuchinoue S, Ishii Y, Sawada T, . The 5-year outcome of ABO-incompatible kidney transplantation with rituximab induction. Transplantation. 2011;91:853–857.
- Chung BH, Lee JY, Kang SH, . Comparison of clinical outcome between high and low baseline anti-ABO antibody titers in ABO-incompatible kidney transplantation. Ren Fail. 2011;33:150–158.
- Tobian AA, Shirey RS, Montgomery RA, Tisch DJ, Ness PM, King KE. Therapeutic plasma exchange reduces ABO titers to permit ABO-incompatible renal transplantation. Transfusion. 2009;49:1248–1254.
- Vieira CA, Agarwal A, Book BK, . Rituximab for reduction of anti-HLA antibodies in patients awaiting renal transplantation: 1. Safety, pharmacodynamics, and pharmacokinetics. Transplantation. 2004;77:542–548.
- Toki D, Ishida H, Horita S, Setoguchi K, Yamaguchi Y, Tanabe K. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients. Transpl Int. 2009;22:447–454.
- Shirakawa H, Ishida H, Shimizu T, . The low dose of rituximab in ABO-incompatible kidney transplantation without a splenectomy: A single-center experience. Clin Transplant. 2011;25:878–884.
- Schroder C, Azimzadeh AM, Wu G, Price JO, Atkinson JB, Pierson RN. Anti-CD20 treatment depletes B-cells in blood and lymphatic tissue of cynomolgus monkeys. Transpl Immunol. 2003;12:19–28.
- Lawrence C, Galliford JW, Willicombe MK, . Antibody removal before ABO-incompatible renal transplantation: How much plasma exchange is therapeutic? Transplantation. 2011;92:1129–1133.
- Tobian AA, Shirey RS, Montgomery RA, . ABO antibody titer and risk of antibody-mediated rejection in ABO-incompatible renal transplantation. Am J Transplant. 2010;10:1247–1253.
- Grim SA, Pham T, Thielke J, . Infectious complications associated with the use of rituximab for ABO-incompatible and positive cross-match renal transplant recipients. Clin Transplant. 2007;21:628–632.
- Aikawa A, Ohara T, Arai K, . Clinical outcome and accommodation in ABO incompatible kidney transplantation. Clin Transplant. 2004;135–142.
- Habicht A, Broker V, Blume C, . Increase of infectious complications in ABO-incompatible kidney transplant recipients a single centre experience. Nephrol Dial Transplant. 2011;26:4124–4131.
- Choi BS, Shin MJ, Shin SJ, . Clinical significance of an early protocol biopsy in living-donor renal transplantation: Ten-year experience at a single center. Am J Transplant. 2005;5:1354–1360.
- Solez K, Colvin RB, Racusen LC, . Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753–760.
- Setoguchi K, Ishida H, Shimmura H, . Analysis of renal transplant protocol biopsies in ABO-incompatible kidney transplantation. Am J Transplant. 2008;8:86–94.
- Haas M, Segev DL, Racusen LC, . C4d deposition without rejection correlates with reduced early scarring in ABO-incompatible renal allografts. J Am Soc Nephrol. 2009;20:197–204.
- Montgomery RA, Locke JE, King KE, . ABO incompatible renal transplantation: A paradigm ready for broad implementation. Transplantation. 2009;87:1246–1255.