Abstract
Introduction: Many studies support the role of vitamin D in the pathogenesis of both types of diabetes. Pancreatic tissues express the vitamin D receptor (VDR) and vitamin D-binding protein; some allelic variations in genes involved in vitamin D metabolism and VDR are associated with glucose intolerance, defective insulin secretion, and sensitivity. Epidemiological links have been established between type 2 diabetes mellitus (DM) and hepatitis C virus (HCV) infection. Aim: To explore the possible therapeutic potential of pharmacologic doses of 1-α-hydroxy vitamin D therapy in improving pancreatic β-cell function in HCV seropositive hemodialysis (HD) patients. Patients and methods: Twenty HCV seropositive HD patients and 20 HCV seronegative patients as control group were randomly selected from HD units. 1-α-Hydroxy vitamin D therapy was administrated in the dose ranged from 0.25 to 0.5 μg/day for 3 months. Corrected total serum calcium, phosphorus, intact parathyroid hormone (iPTH), 25-hydroxy vitamin D [25(OH) vitamin D], 1,25-dihydroxy vitamin D, and glucoparameters [fasting blood glucose, glycohemoglobin test (HbA1c%), homeostatic model assessment (HOMA)-insulin resistance, and HOMA-β-cell function% (B%)] were measured under basal conditions and after 3 months of therapy. Results: There was highly significant improvement in the concentrations of fetal bovine serum (FBS), serum insulin, HbA1c%, 25(OH) vitamin D, and HOMA-β-cell function in HCV seropositive and HCV seronegative groups after oral 1-alphacalcidiol therapy (p < 0.001). Positive correlation exists between the percentage increase in serum insulin and that in HOMA-β-cell function versus 25(OH) vitamin D (p < 0.021 and p < 0.027, respectively) in HCV negative group. Conclusion: 1-α-Hydroxy vitamin D oral therapy may improve glycemic control in HCV seropositive and HCV seronegative HD patients.
INTRODUCTION
Previous studies have documented that vitamin D deficiency is common in the general population and even more common in patients on dialysis.Citation1–4 Recent evidence in the pathogenesis of type 2 diabetes milletus (DM) suggests that alterations in vitamin D status may affect insulin sensitivity, β-cell function, or both, given the discovery of vitamin D receptors (VDRs) in β cells and vitamin D-dependent calcium-binding proteins in pancreatic tissue.Citation5 Vitamin D is essential for normal insulin release in response to glucose and for maintenance of glucose tolerance, whereas deficiency results in decreased insulin secretion without altering glucagon secretion. Pittas et al.Citation6 have summarized the biological evidence implicating a potential influence of vitamin D on glucose homeostasis. The inferences for the manifold roles of vitamin D include the presence of specific VDRs on pancreatic β cells,Citation7 the expression of 1-α-hydroxylase enzyme in pancreatic β cells that catalyzes the conversion of 25(OH) vitamin D to 1,25-dihydroxy vitamin D [1,25(OH)2D],Citation8 the presence of a vitamin D response element in the human insulin gene promoter,Citation9 and the presence of VDR in skeletal muscle.Citation10 In addition, 25(OH)2 vitamin D directly activates transcription of the human insulin receptor gene,Citation11 activates peroxisome proliferator activator receptor-δ,Citation12 stimulates the expression of insulin receptor, and enhances insulin-mediated glucose transport in vitro.Citation13 In patient on dialysis, impaired glycemic control is highly prevalent and is associated primarily with an increase in target tissue insulin resistance.Citation14 Insulin resistance in normoglycemic adults with normal renal function has been shown to be improved with vitamin D supplementation.Citation15 It was reported that vitamin D deficiency might play a major role in the pathogenesis of impaired glucose metabolism in uremia,Citation16 and some small studies on HD patients have reported improvement in insulin resistance using 1,25(OH)2 vitamin D3 or its analogs for 4–26 weeks.Citation17–21 Vitamin D studies in patients on dialysis have predominantly used active vitamin D compounds and focused on 1,25(OH)2 vitamin D3 deficiency. It is not clear whether there is a direct relation between serum 25(OH) vitamin D levels and insulin resistance and pancreatic β-cell function in HCV-positive patients on HD. Several studies have also reported that HCV infection may also contribute to the development of DM and higher prevalence of type 2 DM has been observed in patients with HCV infection than in those with other forms of chronic hepatitis.Citation22–24 HCV infection has been shown to have direct and/or indirect effects on glucose metabolism, leading to insulin resistance and, in predisposed individuals, type 2 diabetes. The mechanism of pathogenesis of diabetes in patients with HCV infection remains unclear, although it has been suggested that insulin resistance plays an important role.Citation25–27 The mechanism through which HCV is associated with insulin resistance involves direct viral effects, proinflammatory cytokines, and suppressors of cytokine signaling.Citation28–30 The detailed molecular events leading to insulin resistance in HCV-infected patients are unclear. HCV infects primarily the liver and, to a very minor extent, mononuclear cells. Direct interactions between the HCV products and the hepatocyte insulin signaling pathway have been reported by several authors. However, recent evidence supports the existence of a significant extrahepatic component of HCV-induced insulin resistance.Citation24 Although there is a growing body of literature on the link between type 2 DM and HCV, the studies are contradictory and the data are inconclusive.Citation25 In the hepatic parenchyma, vitamin D3 is converted by one of several high-capacity cytochrome P450s to 25(OH) vitamin D3; the microsomal CYP2R1 appears to have the highest affinity for substrate vitamin D.Citation31 The most plentiful and stable metabolite of vitamin D in human serum is 25(OH) vitamin D.Citation32 This study aimed to explore the possible therapeutic potential for pharmacologic doses of 1-α-hydroxy vitamin D therapy in improving insulin resistance and pancreatic β-cell function in HCV seropositive prevalent HD patients.
SUBJECTS AND METHODS
A prospective observational cohort study was performed on 20 HCV seropositive HD patients and 20 HCV seronegative patients as control group. Both groups were randomly selected from an HD unit. The original renal disease was diabetic nephropathy (n = 15), hypertensive nephrosclerosis (n = 15), chronic glomerulonephritis (n = 10), and chronic pyelonephritis (n = 5). The renal diagnosis was unknown in 15 patients. Patients with severe hyperphosphatemia (>7 mg/dL) or high calcium/phosphate solubility product (>70) were excluded from the study. All patients were instructed to stop the prescribed vitamin D therapy as washout period for 1 month before the study. 1-α-calcidol (LEO Pharma product, Copenhagen, Denmark) was given in conventional doses (range from 0.25 to 0.5 μg/day) to 40 HD patients whose 25(OH) vitamin D levels were seriously deficient (<12 ng/mL) at the start of the study. The subsequent doses were adjusted according to the levels of plasma calcium, phosphorus, calcium–phosphorus product, and parathyroid hormone (PTH) as follows: 1-α-calcidol treatment was given as 0.5 μg/day for patients with serum calcium level <9.5 mg/dL and intact PTH (iPTH) level >300 pg/mL; if serum calcium level was 9.5–10.5 mg/dL or iPTH level was 120–300 pg/mL, 1-α-calcidol treatment was reduced to 0.25 μg/day. 1-α-calcidol treatment was held if serum calcium level was >11 mg/dL, serum phosphorus level >7 mg/dL, calcium–phosphorus product >60, or PTH level <120 pg/mL. Insulin resistance, β-cell function, 25(OH) vitamin D, and 1,25(OH) vitamin D were measured at the start of the study and again after 12 weeks of oral administration of 1-α-calcidol. No randomized controlled trial could be carried out since neither a placebo group nor the initiation of 1-α-calcidol therapy in severely vitamin D-deficient patients could be justified. This study was performed in accordance with the international standards of good clinical practice and the World Medical Association Declaration of Helsinki and subsequent amendments, and approved by our institute ethical committee. Meanwhile, written informed consent was obtained from all patients.
BLOOD SAMPLING AND ASSAYS
Blood was taken in the morning after an overnight fasting. Whole blood was used for hematocrit, ethylenediaminetetraacetic acid (EDTA) plasma for glucose, insulin, and serum for other biochemical assays. Glucose was measured by a glucose oxidase method. Insulin was measured by solid-phase enzyme-linked immunosorbent assay (DRG® Insulin Enzyme Immunoassay kits; DRG International Inc., NJ, USA). iPTH levels were determined by enzyme-amplified sensitivity immunoassay (Roche Diagnostics, Indianapolis, IN, USA). 1,25(OH) Vitamin D was measured by a competitive enzyme immunoassay (EIA) technique with a selected monoclonal antibody recognizing 1,25(OH)2 vitamin D (DRG® 1,25(OH) Vitamin D Enzyme-Linked-Immuno-Sorbent-Assay kits; DRG International Inc.). 25(OH) Vitamin D was measured by a competitive protein-binding enzyme-linked immunosorbent assay (ELISA); it is based on the competition of 25(OH) vitamin D present in the sample with 25(OH) vitamin D tracer, for the binding pocket of vitamin D-binding protein (DRG® 25(OH) Vitamin D ELISA, USA). The reference range of 25(OH) vitamin D is as follows: seriously deficient <12 ng/mL, insufficiency (deficient) 12–30 ng/mL, and sufficiency >30 ng/mL. The reference range of 1,25(OH) vitamin D in healthy adults was 17–53 pg/mL. Other routine assays (e.g., calcium and phosphate) were made using standard autoanalyzer methodology. Insulin resistance was assessed using the homeostatic model assessment (HOMA) formula originally described by Mathew et al.; HOMA-insulin resistance (IR) index equation [fasting glucose (mg/dL) × fasting insulin (μU/mL)/405] and β-cell function was assessed according to HOMA-B% equation [20 × fasting insulin (μU/mL)/FBG (mg/dL) − 63], respectively, at the onset and after 1-α-calcidol therapy was given for 3 months.Citation33
STATISTICAL ANALYSIS
Data were analyzed using the SPSS version 15. Quantitative data are presented using mean and standard deviation. The median and range were used for data with extreme values. t-Tests for paired and independent samples were used for comparison of quantitative data with normal distribution and the equivalent nonparametric tests (Wilcoxon and Mann–Whitney) were used for non-normally distributed data. Spearman’s correlation was used for bivariate analysis. Multiple linear regression was used to control confounding predictors after logarithmic transformation for skewed data to demonstrate the relationships between 25(OH) vitamin D and glucoparameters.
RESULTS
Twenty patients were found to be suffering from type 2 DM (9 diabetic patients in HCV seropositive HD group and 11 diabetic patients in HCV seronegative group), 13 diabetic patients were receiving small doses of oral hypoglycemic agents (glipizide 2.5–5 mg/day; product of Pharmacia Italia S.P.A. Gruppo Pfizer Inc., Nerviano, Italy) and maintained on the same therapy with no change in dosage throughout the study, and 7 patients were under diet control. Among our selected subjects, nondiabetic patients were also included in this study as insulin resistance in normoglycemic adults has been shown in previous studies to be improved with vitamin D supplementation.Citation15,34 Mean doses of 1-α-calcidol given to HCV seropositive patients versus HCV seronegative patients were 0.40 and 0.37 μg, respectively (p > 0.05), whereas mean values in diabetic versus nondiabetic patients were 0.44 and 0.40 μg, respectively (p > 0.05). Our study showed no significant difference between the HCV seropositive HD patients and the HCV seronegative HD patients regarding age, gender, and body mass index (BMI); meanwhile, there was significant difference in dialysis duration (). After 3 months of 1-α-calcidol therapy, there was significant increase in the mean value of fasting blood glucose, fasting insulin, 25(OH) vitamin D, and median of HOMA-β-cell function, and decrease in glycohemoglobin test (HbA1c%) in both groups (p < 0.001). Also at the end of therapy, the level of 1,25(OH) vitamin D increased significantly in HCV seropositive group only (p = 0.007; and ). Although the level of 25(OH) vitamin D improved after 1-α-calcidol therapy, its mean level was still deficient, that is, <30 ng/mL, especially in HCV seropositive patients with chronic liver disease due to partial defect in the hydroxylation of 1-α-hydroxy vitamin D in the liver. The levels of 25(OH) vitamin D after 1-α-calcidol therapy were seriously deficient (<12 ng/mL) in 3.03% of HD patients, deficient (12–30 ng/mL) in 84% of patients, and sufficient (>30 ng/mL) in 12.12% of the patients. There was no significant difference in percentage change of fetal bovine serum (FBS), serum insulin, 25(OH) vitamin D, 1,25(OH) vitamin D, HOMA-IR, HOMA-β-cell function, HBA1c, and iPTH between the two groups before and after therapy. Our study showed positive correlation between the increase of serum insulin and the increase of HOMA-β-cell function versus 25(OH) vitamin D in HCV seronegative patients (p < 0.021 and p < 0.027, respectively; and ), whereas no significant correlation (p > 0.05) was found in HCV seropositive patients ( and ). No significant correlation was also found between high-sensitive C-reactive protein (hsCRP) and both 25(OH) vitamin D and 1,25(OH) vitamin D (p > 0.05), and between hsCRP and glucoparameters (p > 0.05). Multiple regression analysis shows no independent predictors of any glucoparameters. Comparing vitamin D levels between nondiabetic patients and diabetic patients at the start of the study showed no significant difference in the mean concentration of 25(OH) vitamin D or 1,25(OH) vitamin D. Meanwhile, at the end of study, a significant difference was found in the mean concentration of 25(OH) vitamin D (19.73 ± 6.72 ng/mL) in nondiabetic versus (26.64 ± 7.75 ng/mL) in diabetic patients (p = 0.007); therefore, our study showed significant increase in the mean concentrations 25(OH) vitamin D in diabetic patients by the end of therapy.
Figure 1. Correlation between the percentage of change in serum insulin and 25-hydroxy vitamin D in HCV seronegative group.
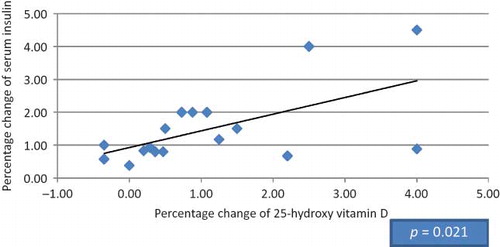
Figure 2. Correlation between the percentage of change in serum insulin and 25-hydroxy vitamin D in HCV seropositive group.
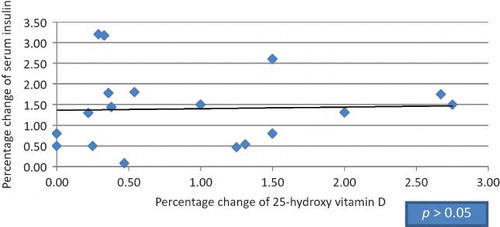
Figure 3. Correlation between the percentage of change in HOMA-B% and 25-hydroxy vitamin D in HCV seronegative group.
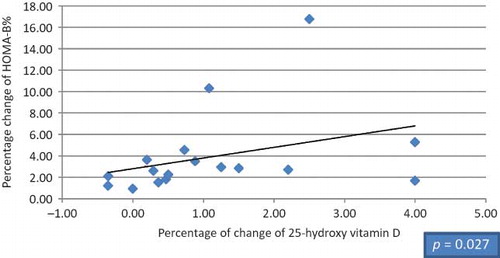
Figure 4. Correlation between the percentage of change in HOMA-B% and 25-hydroxy vitamin D in HCV seropositive group.
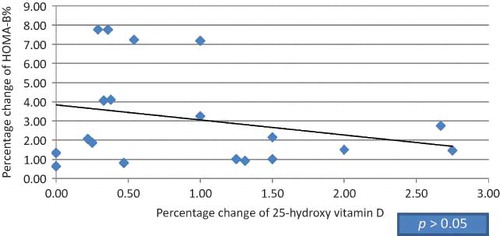
Table 1. Comparison of the change in laboratory characteristics between the start and the end of the study in patient with HCV-negative antibodies.
Table 2. Comparison of the change in laboratory characteristics between the start and the end of the study in patient with HCV-positive antibodies.
Table 3. Comparison of the percentage change of vitamin D, iPTH, and glucoparameters in both groups.
DISCUSSION
Glucose intolerance, insulin resistance, and hyperinsulinemia are common findings in patients on dialysis. Several factors such as uremic toxins, exercise intolerance, metabolic acidosis, secondary hyperparathyroidism, and vitamin D deficiency are implicated in the pathogenesis of insulin resistance in chronic kidney disease.Citation14,35 There are several plausible mechanisms by which the vitamin D status may affect insulin sensitivity. First, decreased vitamin D concentrations result in elevated concentrations of PTH. Elevated PTH in turn affects insulin sensitivity by regulating the intracellular free calcium concentrations in cells like pancreatic β cells.Citation36,37 Studies have shown that increased PTH concentrations were associated with impaired glucose tolerance and decreased insulin sensitivity independent of vitamin D status.Citation38,39 The role of elevated PTH levels in insulin secretion is controversial. Several studies reported that patients with primary hyperparathyroidism are insulin resistant and hyperinsulinemic.Citation5,25–27 Second, vitamin D may play a role in insulin action by stimulating the expression of insulin receptor, thereby enhancing insulin responsiveness for glucose transport.Citation9,13 Calcitriol increases insulin secretion in many ways, including increasing intracellular calcium that is essential for two of the four proteases that release insulin from proinsulin.Citation40 Finally, vitamin D has a modulating effect on the immune system.Citation41,42 Poorer vitamin D status might induce a higher inflammatory response, which is associated with insulin resistance.Citation43,44 Several clinical intervention studies in patients with normal renal function reported that vitamin D, or its active metabolite 1,25(OH)2 vitamin D3, improves insulin sensitivity, even in patients with glucose metabolism parameters within normal ranges.Citation2 The mechanisms, which may lead to this effect, include potential relationships with improvements in lean mass, altered insulin receptor expression, and specific effects on insulin action. It was reported that these actions may be mediated by systemic or local production of 1,25(OH)2 vitamin D3 or by suppression of PTH.Citation2 Apart from changes in the electrolytes, the secondary hyperparathyroidism, vitamin D deficiency, is supposed to play a major role in the pathogenesis of uremic glucose intolerance and insulin resistance.Citation16 These conflicting results concerning the role of secondary hyperparathyroidism and/or vitamin D deficiency in uremia stimulated this study. Kautzky-WillerCitation20 investigated the effect of a 12-week intravenous treatment with 1,25-dihydroxycholecalciferol on glucose metabolism in 10 HD patients. These authors concluded that intravenous calcitriol treatment led to a significant reduction in PTH levels and to a complete normalization of insulin sensitivity in the HD patients, acting either directly or by reducing secondary hyperparathyroidism.Citation14,45,46 Several studies have shown that intravenous calcitriol supplementation leads to about fourfold higher plasma peak responses compared with the oral preparation. Intravenous treatment to oral preparation yields a more efficient suppression of parathormone secretion and allows a greater delivery to peripheral tissues, which might increase important biological effects of the vitamin.Citation47 All target tissues for vitamin D activate it in situ, thus giving intact vitamin D (cholecalciferol) may prove more effective replacement than using calcitriol or its analogs, even in renal failure, a concept currently under active investigation. This study showed a significant increase in 25(OH) vitamin D level in both groups after oral 1-α-hydroxy vitamin D therapy (p < 0.001). Quesada et al. reported that serum calcitriol values, which were low as expected, increased after 14 days of treating HD patients with oral calcitriol (0.5 μg/day), but did not reach the values observed in healthy controls. Similar to our study, there was no placebo group in his study. Also, an enhancement in insulin secretion following calcitriol was observed (p < 0.001).Citation48 Chiu et al. found that insulin sensitivity is improved by as much as 60% when the levels of vitamin D are increased from 25 nmol/L to 75 nmol/L.Citation2,49 Few studies found that intravenous 1,25(OH) vitamin D3 therapy corrected glucose intolerance, insulin resistance, and hypoinsulinemia in patients on HD whether PTH was suppressed.Citation19–21 This is the first study in HCV seropositive patients to investigate the effect of oral 1-α-hydroxy vitamin D therapy on glucose homeostasis, as most studies utilize calcitriol either orally or intravenously. In contrast, our study showed that HOMA-IR did not improve after oral 1-α-calcidol therapy that may be due to short duration of therapy, absence of change in BMI, oral rather than intravenous therapy, and failure of suppression of iPTH level after therapy. Although the mean 25(OH) vitamin D level increased after therapy in HCV seropositive and HCV seronegative patients, it is still below the sufficiently adequate level of 25(OH) vitamin D that should be more than 30 ng/mL. However, our study showed that serum insulin level and HbA1c improved in both groups after therapy (p < 0.001). This change is not attributed to PTH changes as PTH median level increased after therapy from 82 (52–850) to 195 pg/mL (20–1333) in HCV seronegative patients and from 84 (28–806) to 269 pg/mL (39–1445) in HCV seropositive patients after therapy (p > 0.05). It seems reasonable that factors other than hyperparathyroidism in patients with vitamin D deficiency, including VDR polymorphism or sensitivity of muscle receptor of vitamin D, may affect insulin resistance.Citation50 The improvement in HOMA-β-cell function, fasting insulin, could be the fact that pancreatic tissue (more specifically the insulin-producing β cells) expresses the VDR and vitamin D-binding protein. Various animal studies showed that the endocrine pancreas is a target for the active metabolite of vitamin D and that this metabolite seems to play a major role in the modulation of insulin secretion.Citation51,52 It has been shown that dietary vitamin D repletion significantly increased insulin secretion and vitamin D deficiency was associated with an inhibition of pancreatic insulin release.Citation53 In another study done by Kadowski and Norman, it was found that 1,25(OH)2 vitamin D3, but not other vitamin D metabolites, seems to play an essential role in the modulation of insulin secretion.Citation54 This work showed a significant improvement in blood glucose control where HbA1c decreased in both groups after therapy (p < 0.001), and this goes with Strózecki et al.Citation18 who investigated the influence of intravenous 1,25(OH)2 vitamin D3 therapy on the long-term control of glycemia in hemodialyzed patients with severe secondary hyperparathyroidism. They found that intravenous 1,25(OH)2 vitamin D3 therapy is associated with the decrease in HbA1c even in patients with severe secondary hyperparathyroidism. This may indicate a positive impact of intravenous 1,25(OH)2 vitamin D3 therapy on the long-term glucose metabolism. Our study, similar to Luo et al., showed a lack of a relationship between vitamin D and hsCRP as inflammatory marker.Citation55 There is evidence from in vitro studies that vitamin D may modulate inflammatory cytokines, and thus affect insulin sensitivity and β-cell survival; however, there are few studies that report on inflammatory markers in relation to vitamin D status in diabetes. In a placebo-controlled study by Pittas et al.,Citation15 combined calcium and vitamin D supplementation in those with impaired fasting glucose did not alter hsCRP or interleukin-6 levels. It is likely therefore that vitamin D may not exert its immunomodulatory effect via these molecules.
This prospective, single-center analysis has various shortcomings: the relatively small size of the study population, low doses of oral rather than intravenous vitamin D therapy, and the short duration of follow-up. Also, we do not know the hepatitis C viral loads of the patients as this was not assessed.
CONCLUSION
Patients with hypovitaminosis D are at higher risk of insulin resistance even if they are treated with the activated form of vitamin D for hyperparathyroidism. 1-α-calcidol treatment can improve fasting blood sugar, HbA1c, and HOMA-β-cell function in both HCV seropositive and HCV seronegative patients. This therapy has potential for reducing the risks of type 2 DM in HD patients who are also HCV seropositive. Prospective studies are needed to determine whether long-term supplementation with 1-α-calcidol in HD patients can maintain reduced insulin resistance and reduce type 2 DM risks in those who are HCV seropositive.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Tangpricha V, Pearce EN, Chen TC, . Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659–662.
- Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820.
- Mertens PR, Muller R. Vitamin D and cardiovascular risk. Int Urol Nephrol. 2010;42(1):165–171.
- Rammos G, Tseke P, Ziakka S, Vitamin D, The renin-angiotensin system, and insulin resistance. Int Urol Nephrol. 2008;40(2):419–426.
- Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, . Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:185–197.
- Pittas AG, Lau J, Hu FB, Dawson-Hughes. B. The role of vitamin D and calcium in type 2 diabetes: A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6): 2017–2029.
- Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol. 1994;267(3):356–360.
- Bland R, Markovic D, Hills CE, . Expression of 25-hydroxyvitamin D3-1α-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89–90:121–125.
- Maestro B, Davila N, Carranza MC, Calle C. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84(2–3):223–230.
- Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260(15):8882–8891.
- Maestro B, Molero S, Bajo S, Dávila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D3. Cell Biochem Funct. 2002;20(3):227–232.
- Dunlop TW, Väisänen S, Frank C, . The human peroxisome proliferator-activated receptor δ gene is a primary target of 1alpha, 25‐dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol. 2005;349(2):248–260.
- Maestro B, Campion J, Davila N, Calle. C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocrine J. 2000;47(4):383–391.
- DeFronzo RA, Alvestrand A, Smith D, . Insulin resistance in uremia. J Clin Invest. 1981;67:563–568.
- Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986.
- Mak RHK. Amelioration of hypertension and insulin resistance by 1,25-dihydroxycholecalciferol in hemodialysis patients. Pediatr Nephrol. 1992;6:345–348.
- Mak RHK. Intravenous 1,25 dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int. 1992;41:1049–1054.
- Strózecki P, Kretowicz M, Odrow-Sypniewska G, Manitius J. The influence of intravenous 1, 25(OH)2D3 therapy on glucose metabolism in hemodialyzed patients with secondary hyperparathyroidism. Ren Fail. 2004;26:345–348.
- Blair D, Byham-Gray L, Lewis E, McCaffrey S. Prevalence of vitamin D [25(OH)D] deficiency and effects of supplementation with ergocalciferol (vitamin D2) in stage 5 chronic kidney disease patients. J Ren Nutr. 2008;18:375–382.
- Kautzky-Willer A, Pacini G, Barnas U, . Intravenous calcitriol normalizes insulin sensitivity in uremic patients. Kidney Int. 1995;47:200–206.
- Wolf M, Shah A, Gutierrez O, . Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013.
- Bernsmeier C, Heim MH. Insulin resistance in chronic hepatitis C: Mechanisms and clinical relevance. Swiss Med Wkly. 2009;139:678–684.
- Lonardo A, Adinolfi LE, Petta S, Craxì A, Loria P. Hepatitis C and diabetes: The inevitable coincidence? Expert Rev Anti Infect Ther. 2009;7:293–308.
- Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537–1547.
- Konrad T, Zeuzem S, Vicini P, . Evaluation of factors controlling glucose tolerance in patients with HCV infection before and after 4 months therapy with interferon-a. Eur J Clin Invest. 2000;30(2):111–121.
- Petit JM, Bour JM, Galland-Jos C, . Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. Hepatology. 2001;35:279–283.
- Chen LK, Hwang SJ, Tsai ST, . Glucose intolerance in Chinese patients with chronic hepatitis C. World J Gastroenterol. 2003;9:505–508.
- Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: A mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384–1392.
- Kawaguchi T, Ide T, Taniguchi E, . Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570–576.
- Douglas MW, George J. Molecular mechanisms of insulin resistance in chronic hepatitis C. World J Gastroenterol. 2009;15:4356–4364.
- Strushkevich N, Usanov SA, Plotnikov AN, . Structural analysis of CYP2R1 in complex with vitamin D3. J Mol Biol. 2008;380:95–106.
- Chun RF, Adams JS, Hewison M. Back to the future: A new look at ‘old’ vitamin D. J Endocrinol. 2008;198:261–269.
- Matthews DR, Hosker JP, Rudenski AS, . Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419.
- Von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient: A randomized, placebo-controlled trial. Br J Nutr. 2010;103(4):549–555.
- Türk S, Yeksan M, Tamer N, Gürbilek M, . Effect of 1,25 (OH)2 D3 treatment on glucose intolerance in uremia. Nephrol Dial Transplant. 1992;7:1207–1212.
- Naveh-Many T, Silver J. Vitamin D and the Parathyroid. 2nd ed. London: Elsevier; 2004.
- Christakos S, Gabrielides C, Rhoten WB. Vitamin D-dependent calcium binding proteins: Chemistry, distribution, functional considerations, and molecular biology. Endocr Rev. 1989;10:3–26.
- Chiu KC, Chuang LM, Lee NP, . Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism. 2000;49:1501–1505.
- Reis JP, von Muhlen D, Kritz-Silverstein D, . Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–1555.
- Davidson HW, Rhodes CJ, Hutton JC. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988;333(6168):93–96.
- Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007;103: 558–562.
- Cantorna MT, Mahon BD. D-hormone and the immune system. J Rheumatol Suppl. 2005;76:11–20.
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180.
- Mlinar B, Marc J, Janez A, Pfeifer M. Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta. 2007;375:20–35.
- Gunal AI, Celı’ker H, Celebı’ H, . Intravenous alfacalcidol improves insulin resistance in hemodialysis patients. Clin Nephrol. 1997;48:109–113.
- Lin S, Lin Y, Lu K, . Effects of intravenous calcitriol on lipid profiles and glucose tolerance in uremic patients with secondary hyperparathyroidism. Clin Sci. 1994;87:533–538.
- Rodriguez M, Felsenfeld AJ, Williams C, . The effects of long-term intravenous calcitriol administration on parathyroid function in hemodialysis patients. J Am Soc Nephrol. 1991;2:1014–1020.
- Quesada JM, Martin-Malo A, Santiago J, . Effect of calcitriol on insulin secretion in uremia. Nephrol Dial Transplant. 1990;5(12):1013–1017.
- Inzucchi SE, Maggs DG, Spollett GR, . Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338(13):867–872.
- Bonakdaran S, Ayatollahi H, Mojahedi MJ, . Impact of treatment with oral calcitriol on glucose intolerance and dyslipidemia(s) in hemodialysis patients. Saudi J Kidney Dis Transpl. 2008;19(6):942–947.
- Roth J, Bonner weir S, Norman AW, . Immunohistochem-istry of vitamin D dependent calcium binding protein in chick pancreas exclusive localization in beta cells. Endocrinology. 1982;116:2216–2218.
- Christakos S, Norman AW. Studies on the mode of action of calciferol: Biochemical characterization of 1,25 dihydroxyvitamin D3 receptors in chick pancreas and kidney cytosol. Endocrinology. 1981;108:140–149.
- Norman AW, Frankel BJ, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825.
- Kadowski S, Norman AW. Demonstration that the vitamin D3 metabolite 1,25(OH)2 vitamin D3 and not 24R, 25(OH)2 vitamin D3 is essential for normal insulin secretion in the perfused rat pancreas. Diabetes. 1985;34:315–321.
- Luo C, Wong J, Brown M, Hooper M, Molyneaux L, Yue DK. Hypovitaminosis D in Chinese type 2 diabetes: Lack of impact on clinical metabolic status and biomarkers of cellular inflammation. Diab Vasc Dis Res. 2009;6(3):194–199.
