Abstract
Vitamin D deficiency is common globally. There is evidence that vitamin D status may be related to immune function and cardiovascular disease. The vitamin D status of Chinese kidney transplant recipients has never been investigated. We performed a cross-sectional study and measured the level of 25-hydroxyvitamin D [25(OH)D] in 94 Chinese renal transplant recipients with stable allograft function. Vitamin D deficiency and insufficiency were detected in 43.6% and 54.2% of patients, respectively. About 53.2% of the patients also had elevated parathyroid hormone (PTH) levels. The level of 25(OH)D was lower in kidney transplant recipients compared with healthy controls matched for age and sex (52.5 ± 15.6 nmol/L vs. 57.5 ± 19.0 nmol/L, p = 0.05), but the level of serum creatinine was higher in kidney transplant recipients (120.3 ± 48.5 μmol/L and 78.3 ± 15.3 μmol/L, p < 0.01). The level of 25(OH)D was negatively correlated with that of PTH (p = 0.001). The latter was associated with serum creatinine (p = 0.001) and duration of dialysis (p = 0.001). Patients with a history of acute rejection showed lower levels of 25(OH)D (45.3 ± 11.9 nmol/L vs. 54.2 ± 16.0 nmol/L, p = 0.003). We conclude that vitamin D deficiency is prevalent among Chinese renal transplant recipients. In view of the potential immunomodulatory effect of vitamin D, the relationship between vitamin D level and rejection and the effect of vitamin D supplementation in renal transplant recipients warrant further investigations.
INTRODUCTION
Vitamin D deficiency is well known to play a role in the pathogenesis of secondary hyperparathyroidism in patients with chronic kidney disease (CKD).Citation1 It has also been demonstrated that vitamin D deficiency contributed to bone loss in renal transplant recipients and resulted in increased fracture rates and morbidity.Citation2 In addition to its well-established role in bone mineral metabolism, the nonclassical effects of vitamin D on the cardiovascular, renal, and immune systems are gaining increasing attention recently.Citation3 Vitamin D deficiency has demonstrated associations with increased risk of hypertension, cardiovascular disease, glucose intolerance, infectious diseases, autoimmune disorders, and malignancy.Citation4
The data to date suggest that vitamin D insufficiency is common among renal transplant recipients. It was reported that the prevalence of vitamin D insufficiency among stable renal transplant recipients was up to 85% in the United Kingdom,Citation5 and 94.7% among African Americans.Citation6 Vitamin D status shows geographical and racial variation.Citation7 There are no data on the vitamin D status in Chinese renal transplant recipients. With inadequate information on the vitamin D status and its clinical consequences, the management of vitamin D insufficiency in renal transplant recipients remains undefined. We therefore performed a cross-sectional study to investigate the vitamin D status in renal transplant recipients and looked for parameters that were associated with vitamin D status.
MATERIALS AND METHODS
This cross-sectional study was carried out during the period 1 August 2010 to 31 January 2011. Adult renal transplant recipients with stable renal allograft function, defined as <15% fluctuation of serum creatinine over the previous 6 months, more than 1 year after kidney transplantation were recruited from a single center. Patients with acute rejection in the past 6 months, those who had undergone parathyroidectomy, or those who were treated with vitamin D were excluded.
Demographic data, including age, gender, and body weight, were recorded. Clinical data including cause of end-stage renal failure; modality and duration of renal replacement therapy prior to transplantation; and complications such as acute rejection, malignancy, and cardiovascular disease were obtained from the review of patients’ records. Cardiovascular disease was defined as prior history of coronary artery revascularization, documented coronary artery stenosis or myocardial hypoperfusion with imaging, or history of myocardial infarction or acute coronary syndrome with characteristic electrocardiographic changes and elevated cardiac enzyme level.
Fasting blood samples were collected from each patient for the measurement of serum creatinine, calcium, phosphate, parathyroid hormone (PTH), hemoglobin, fasting glucose, lipid profile, and 25-hydroxyvitamin D [25(OH)D] levels. The level of 25(OH)D was measured by radioimmunoassay using a kit (DiaSorin, Inc., Stillwater, MN, USA). Vitamin D deficiency was defined as serum 25(OH)D level below 50 nmol/L. The level of 25(OH)D over 75 nmol/L indicated sufficient vitamin D status, and levels in the range 50–75 nmol/L were regarded as vitamin D insufficiency according to established criteria.Citation4 Age- and sex-matched healthy individuals from the general population with no documented renal disease were included as controls.
Data were analyzed using SPSS for Windows software (version 16.0). Continuous data were expressed as mean ± standard deviation. Comparison between groups was performed by chi-square or Fisher’s exact test for categorical data and Student’s t-test, Mann–Whitney U-test, or analysis of variance (ANOVA) as appropriate for continuous data. Continuous variables that were not in normal distribution were logarithmically transformed. Pearson’s correlation testing was used to look for associations between different parameters. A p-value <0.05 was considered statistically significant.
RESULTS
A total of 94 kidney transplant recipients were included in the study (mean age: 51.0 ± 8.8 years; duration of transplantation: 148.7 ± 92.1 months). All were local Chinese. In addition, 94 age- and sex-matched healthy individuals were included as controls (). There were no differences in comorbidities between renal transplant recipients and controls, such as ischemic heart disease (21.3% vs. 28.4%, p = NS) and diabetes (21.3% vs. 23.4%, p = NS). Serum creatinine level was higher in kidney transplant recipients than that in controls (120.3 ± 48.5 μmol/L and 78.3 ± 15.3 μmol/L, respectively, p < 0.01). The level of 25(OH)D was 52.5 ± 15.6 nmol/L in kidney transplant recipients and 57.5 ± 19.0 nmol/L in controls (p = 0.05). Serum 25(OH)D level was similar between patients and controls with estimated glomerular filtration rate (eGFR) of 60 mL/min or above (i.e., CKD stage 1 or 2; ). There were too few controls with more severe degrees of renal impairment for meaningful comparison.
Figure 1. The level of 25(OH)D in renal transplant recipients and healthy controls matched for age, sex, and renal function. The numbers in the columns indicate the number of patients in each subgroup, and the error bars indicate the respective standard deviations.
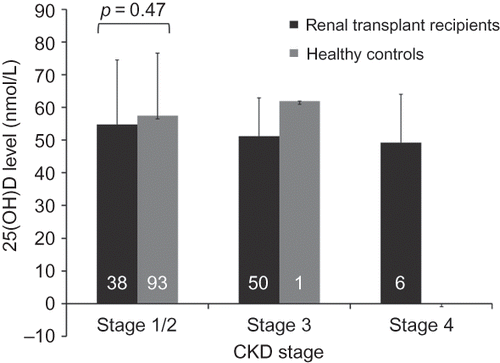
Table 1. Laboratory parameters of 94 renal transplant recipients compared with age- and sex-matched healthy controls.
About 21.3% of samples were taken in the sunny summer months of August–September. The level of 25(OH)D measured in summer and in winter (October–January) did not differ significantly (56.4 ± 21.4 nmol/L vs. 51.3 ± 13.2 nmol/L, p = NS). About 43.6% of kidney transplant recipients had 25(OH)D levels below 50 nmol/L (i.e., vitamin D deficient) and 54.3% had levels in the range 50–75 nmol/L (i.e., vitamin D insufficient). Only 2 (2.1%) patients had sufficient vitamin D status as indicated by 25(OH)D levels above 75 nmol/L. Vitamin D-deficient patients were younger (48.7 ± 10.1 vs. 52.9 ± 7.4 vs. 52.5 ± 0.7 in 25(OH)D < 50 nmol/L, 50–75 nmol/L, and >75 nmol/L group, respectively) and received renal transplant at younger age (36.8 ± 12.1 vs. 40.4 ± 10.6 vs. 45.0 ± 1.4 in 25(OH)D < 50 nmol/L, 50–75 nmol/L, and >75 nmol/L group, respectively; ). However, 25(OH)D level was not significantly associated with age (p = 0.14) and age at transplantation (p = 0.29) on correlation analysis. Duration of dialysis prior to transplantation and duration of follow-up after transplantation did not differ significantly among patients with different vitamin D status.
Table 2. Comparison between kidney transplant recipients with 25(OH)D level <50 nmol/L, 50–75 nmol/L, or >75 nmol/L.
Table 3. Correlation between 25(OH)D and various clinical and laboratory parameters in 94 renal transplant recipients.
PTH level in kidney transplant recipients was 10.2 ± 7.4 pmol/L. Fifty (53.2%) patients had PTH levels above the normal upper limit of 7.3 pmol/L. Calcium and phosphate levels were 2.38 ± 0.11 mmol/L and 1.03 ± 0.16 mmol/L, respectively. The level of 25(OH)D was negatively associated with log-PTH (R = −0.34, p = 0.001), and the latter was significantly associated with log-creatinine (R = −0.33, p = 0.001) and duration of dialysis (R = 0.35, p = 0.001). However, there was no significant correlation between 25(OH)D level and log-creatinine (R = −0.052, p = 0.62), calcium (R = 0.15, p = 0.15), phosphate (R = −0.057, p = 0.58), duration of dialysis (R = −0.074, p = 0.51), and duration of transplantation (R = 0.034, p = 0.74; ).
Eighteen patients had history of acute rejection; the rejection episodes were 148 ± 95 months prior to the measurement of vitamin D level. These patients had significantly lower serum 25(OH)D level (45.3 ± 11.9 nmol/L) compared with those without acute rejection (54.2 ± 16.0 nmol/L, p = 0.003). The levels of 25(OH)D were similar in patients with (n = 9, 49.3 ± 9.0 nmol/L) or without solid organ tumor (n = 85, 52.9 ± 16.2 nmol/L, p = 0.52). The tumors included renal cell carcinoma (n = 4), carcinoma of colon (n = 2), hepatocellular carcinoma (n = 1), carcinoma of ovary (n = 1), and carcinoma of corpus (n = 1). The level of 25(OH)D did not differ between patients with (n = 20, 56.2 ± 14.0 nmol/L) or without (n = 74, 51.5 ± 16.0 nmol/L) cardiovascular disease (p = 0.24).
DISCUSSION
Vitamin D insufficiency is common among renal transplant recipients. Previous studies reported that 85% of Caucasian and 94.7% African American renal transplant recipients had insufficient serum 25(OH)D level.Citation5,6 However, these studies did not include healthy controls for comparison. In fact, there is evidence that vitamin D deficiency is a global health problem. It has been estimated that 1 billion people have vitamin D deficiency worldwide.Citation4 In Hong Kong, over 60% of community dwelling Chinese adult have 25(OH)D level below 75 nmol/L.Citation8,9 In this study, over 90% Chinese renal transplant recipients had insufficient 25(OH)D level. There was no significant difference in the 25(OH)D level between renal transplant patients and healthy controls when stratified according to the stage of CKD. Many factors may contribute to the high prevalence of vitamin D insufficiency in southern Chinese.Citation8,9 These include a low level of outdoor physical activity, use of sunscreen, and the Chinese diet that is low in calcium content and lacks vitamin D-rich food items.
Additional risk factors for vitamin D insufficiency include long-term treatment with corticosteroids that activate gene expression of enzymes that catabolize vitamin D.Citation3 In addition, elevated level of fibroblast growth factor 23 (FGF-23), a phosphatonin that suppresses 25(OH)D, has been reported after kidney transplantation, especially in the early posttransplant period.Citation10 However, the majority of patients included in this study were not in the early posttransplant period and were on low maintenance dose of immunosuppressive medications. Hence, the effect of corticosteroids and FGF-23 should be minimal. This may account for the comparably low 25(OH)D level between the kidney transplant recipients and the healthy controls that were matched for age, sex, and renal function. The inverse relationship between serum 25(OH)D and PTH levels observed in our patients is as expected and consistent with previous reports.Citation5,6 Despite the kidney transplantation, the patients’ PTH level still showed a correlation with the previous duration of dialysis. Although the mean serum creatinine level in kidney transplant patients was 120.3 μmol/L, it showed a correlation with PTH level. In contrast, the level of 25(OH)D was not related to allograft function or dialysis duration. It has been demonstrated in large population study that vitamin D concentrations declined with increasing age.Citation11 Although patients in the vitamin D-deficient and -insufficient categories were younger in this study, there was no significant relationship between age and 25(OH)D level on correlation analysis.
It is of interest to note the lower level of vitamin D in patients with a history of acute rejection, compared with those who did not have any rejection episode. There are data to suggest that vitamin D receptor activation could modulate adaptive immune response and downregulate dendritic cell proliferation and activities.Citation3 Vitamin D receptor agonists (VDRAs) may thus theoretically reduce the risk of allograft rejection, and this immunomodulatory capacity of vitamin D has been observed in several animal models of transplantation.Citation12,13 In the human setting, data from a retrospective case-controlled study suggested that osteoporotic renal transplant recipients experienced fewer episodes of acute rejection after calcitriol treatment.Citation14 However, patients who had acute rejection within 6 months were excluded in our study. Further studies are needed to evaluate the role of vitamin D in acute rejection and the efficacy of VDRA as immunomodulatory agent in renal transplant recipients.
It has been suggested that vitamin D may have a protective effect against the development of cancer. Data from in vitro experiments showed that vitamin D receptor activation induced differentiation and apoptosis and inhibited proliferation and angiogenesis.Citation3 Our data did not show any difference in 25(OH)D level between renal transplant recipients with and without malignancy, although the sample size was small. Epidemiological studies had reported higher prevalence rates of solid organ tumors in individuals living in high latitudes, and decreased vitamin D synthesis was postulated to be a contributing cause.Citation15 Data from case–control studies in the general population have demonstrated an inverse relationship between 25(OH)D level and the incidence of colon breast, prostate, and ovary cancers and lymphoma.Citation3 A higher incidence of posttransplant malignancy has been reported in renal transplant recipients with 25(OH)D level below 25 nmol/L.Citation16
Data from previous studies showed that vitamin D insufficiency may be an independent risk factor for cardiovascular disease. A case–control study demonstrated that patients with prior myocardial infarction had lower vitamin D levels.Citation17 The risk of myocardial infarction in individuals with vitamin D deficiency [25(OH)D < 37.5 nmol/L] was twice of those without vitamin D deficiency [25(OH)D > 75 nmol/L].Citation18 A multiethnic study on atherosclerosis showed that for every 25 nmol/L reduction of 25(OH)D concentration, there was a 23% increased risk for coronary artery calcification (p = 0.049).Citation19 Lower 25(OH)D levels have been demonstrated in patients with congestive heart failure. In addition, vitamin D is a negative regulator of the renin–angiotensin system and an inverse relationship between serum 25(OH)D and blood pressure has been observed.Citation20,21 In contrast to previous studies in general population and dialysis/non-dialysis CKD patients, we could not demonstrate a relationship between serum vitamin D level and cardiovascular disease in renal transplant recipients. The small sample size, in particular the very small number of subjects with “adequate” vitamin D level, the inclusion of only clinically evident cardiovascular disease, and genetic variation were possible confounding factors. Furthermore, the importance of vitamin D in relation to other (more prominent) cardiovascular risk factors is likely to vary between different patient populations.
The limitations of this study include the small sample size and the small number of events of interest such as rejection and malignancy. The cross-sectional design permits examination of association but not causal or temporal relationship. Nevertheless, these original findings highlight the high prevalence of vitamin D deficiency in Chinese renal transplant recipients, the clinical consequence of which warrants further investigations. We also note a high prevalence of hyperparathyroidism despite relatively stable allograft function and long duration of post-transplant follow-up, which could be related to impaired allograft function.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Wolf M, Thadhani R. Beyond minerals and parathyroid hormone: Role of active vitamin D in end-stage renal disease. Semin Dial. 2005;18:302–306.
- Lim WH, Coates PS, Russ GR, Coates PT. Hyperparathyroidism and vitamin D deficiency predispose to bone loss in renal transplant recipients. Transplantation. 2009;88:678–683.
- Courbebaisse M, Souberbielle JC, Thervet E. Potential nonclassical effects of vitamin D in transplant recipients. Transplantation. 2010;89:131–137.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281.
- Stavroulopoulos A, Cassidy MJ, Porter CJ, Hosking DJ, Roe SD. Vitamin D status in renal transplant recipients. Am J Transplant. 2007;7:2546–2552.
- Tripathi SS, Gibney EM, Gehr TW, King AL, Beckman MJ. High prevalence of vitamin D deficiency in African American kidney transplant recipients. Transplantation. 2008;85:767–770.
- Ginde AA, Liu MC, Camargo Jr CA. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632.
- Chan R, Chan D, Woo J, . Serum 25-hydroxyvitamin D and parathyroid hormone levels in relation to blood pressure in a cross-sectional study in older Chinese men. J Hum Hypertens. 2012;26:20–27.
- Wat WZ, Leung JY, Tam S, Kung AW. Prevalence and impact of vitamin D insufficiency in southern Chinese adults. Ann Nutr Metab. 2007;51:59–64.
- Evenepoel P, Naesens M, Claes K, Kuypers D, Tertiary VY. ‘Hyperphosphatoninism’ accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant. 2007;7:1193–1200.
- Scragg R, Sowers M, Serum BC. 25-Hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818.
- Lemire JM, Archer DC, Khulkarni A, Ince A, Uskokovic MR, Stepkowski S. Prolongation of the survival of murine cardiac allografts by the vitamin D3 analogue 1,25-dihydroxy-delta 16-cholecalciferol. Transplantation. 1992;54:762–763.
- Zhang AB, Zheng SS, Jia CK, Wang Y. Effect of 1,25-dihydroxyvitamin D3 on preventing allograft from acute rejection following rat orthotopic liver transplantation. World J Gastroenterol. 2003;9:1067–1071.
- Tanaci N, Karakose H, Guvener N, Tutuncu NB, Colak T, Haberal M. Influence of 1,25-dihydroxyvitamin D3 as an immunomodulator in renal transplant recipients: A retrospective cohort study. Transplant Proc. 2003;35:2885–2887.
- Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–231.
- Ducloux D, Courivaud C, Bamoulid J, Kazory A, Dumoulin G, Chalopin JM. Pretransplant serum vitamin D levels and risk of cancer after renal transplantation. Transplantation. 2008;85:1755–1759.
- Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: A community-based study. Int J Epidemiol. 1990;19:559–563.
- Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch Intern Med. 2008;168:1174–1180.
- de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-Hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–1812.
- Artaza JN, Mehrotra R, Norris KC. Vitamin D and the cardiovascular system. Clin J Am Soc Nephrol. 2009;4:1515–1522.
- Cozzolino M, Ketteler M, Zehnder D. The vitamin D system: A crosstalk between the heart and kidney. Eur J Heart Fail. 2010;12:1031–1041.
