Abstract
The aim of this retrospective study was to evaluate our neonatal intensive care unit (NICU) patients’ characteristics treated with acute peritoneal dialysis (PD) and their risk factors for mortality. We also wanted to share our experience of the application of PD in neonates who required less than 60 mL of dwell volume and their PD-related problems, as well as special solutions for these problems. This study included 27 infants treated in our NICU between February 2008 and December 2011. We retrospectively analyzed these patients’ records. The percutaneous PD catheter was placed by us. PD procedure was performed either by manual technique or automated PD. Statistical evaluation was performed by using χ2-tests and Student’s t-tests. In these 27 neonates, the average gestational age and birth weight were 35.18 ± 4.02 weeks and 2534.62 ± 897.41 g, respectively. The mean PD duration time was 6.11 ± 6.30 days. Of these, 10 patients were treated by manual technique, whereas 17 patients were treated with automated system. Among 27 neonates, 16 patients died. Overall mortality rate was 59.25%. PD-related complications were seen in 25.92% of patients. In conclusion, PD application is less effective and troublesome for low-birth-weight infants. Each center should create its own solutions to accommodate problematic patients in PD treatment to improve the outcome in this special population.
INTRODUCTION
With the advances in care for neonates in neonatal intensive care units (NICUs), the percentages of newborns requiring acute peritoneal dialysis (PD) have been gradually increased. Acute kidney injury (AKI) and other reasons, including inborn errors of metabolism, are the leading indications of acute dialysis in NICUs. Despite continued expertise and/or technological advancement, hemodialysis (HD) cannot be applied effectively in NICU patients. PD has some advantages against HD. HD could not be performed well as neonates have low blood pressure and weak arterial access. PD treatment is relatively easy and technically simple and can be performed even in hemodynamically unstable patients.Citation1 Although PD can be applied in all NICU patients, when necessary, technical manipulations should be put into practice to accommodate smaller infants undergoing dialysis.
The aim of this retrospective study was to evaluate the patient characteristics of neonates treated with acute PD and their risk factors for mortality, with a special focus on these patients treated at our NICU. We also wanted to share our experience of the application of PD in neonates who required less than 60 mL of dwell volume and their PD-related problems, as well as special solutions for these problems.
MATERIALS AND METHODS
Study Population
In this retrospective study, we analyzed the patient records of all neonates treated with acute PD between February 2008 and December 2011 in our NICU. The underlying causes in these 27 neonates (20 boys, 7 girls) were perinatal asphyxia (8), metabolic disease (8), sepsis (4), respiratory distress syndrome (RDS) (1), patent ductus arteriosus (1), coarctation of the aorta (CoA) (1), truncus arteriosus (1), total anomalous pulmonary venous return (TAPVR) (1), posterior urethral valve (PUV) (1), and nephronophthisis (1).
Acute PD was performed in neonates with
| 1. | an abrupt (within 24–48 h) reduction in kidney function with oliguria of less than 0.5 mL/kg/h without distended bladder; | ||||
| 2. | severe edema which is unresponsive to medical treatment; | ||||
| 3. | the signs of uremia (impaired cardiac function or seizures), refractory hyperkalemia, and metabolic acidosis; | ||||
| 4. | fluid overload with respiratory compromise; and | ||||
| 5. | persistent hyperammonemia (blood ammonium level >200 mg/dL) resistant to medical treatment. | ||||
All the parents were informed about the rationale of PD treatment before the procedure.
Catheter Insertion
The percutaneous PD catheter [Tenckhoff, one-cuffed neonatal catheters with straight tips (Quintin® Convidien, Mansfield, MA, USA)] placement was performed by us under local anesthesia in a special incubator. All procedures were carried out under strict aseptic conditions (for doctors: cap, mask, sterile gown, and double sterile gloves; for nurses: cap, mask, and sterile gloves). After the neonates were laid in supine position, the skin was prepared with antiseptic solution (povidone-iodine) 5 times. Under local anesthesia, a 0.5 cm vertical incision through the linea alba 0.5–1.0 cm below the umbilicus was made. Blunt dissection was carried down through the subcutaneous tissue until the rectus sheath was seen. A guided PD catheter was inserted and passed through the peritoneum at an angle of 90° with one breakthrough, then directed caudally toward the left iliac fossa at an angle of 45°. The PD catheter, which was washed with heparinized solution, was threaded on a stiffening stylet and introduced toward the left iliac fossa. The tip of the catheter was placed in the Douglas pouch. After the guide was removed, approximately 25–50 mL of heparinized saline was infused rapidly; its outflow is assessed to confirm adequate function. The PD catheter was fixed by closing the incision with one or two sutures. The titanium adaptor was added to the proximal end of the catheter. The skin was cleaned with antiseptic solution 5 times again. The mupirocin ointment was applied to the catheter exit site followed by covering with a transparent, oxygen-permeable dressing.Citation2
PD Procedure
The PD catheter was connected to a closed system for peritoneal drainage. PD was started with a dwell volume of 10–20 mL/kg. In patients (patient nos 2, 3, 5, 7, 8, 13, 20, 21, and 25) who needed less than 60 mL of dwell volume or who experienced drainage problem in automated system (patient no. 24), installation of PD fluids was carried out using a simple, mechanical, low-tech, and closed system consisting of an infusion pump connected to a Baxter HomeChoice PD Machine (Baxter International Inc., Deerfield, IL, USA) prewarming the PD fluid to 37.1°C. After the dwell, the PD fluid was drained mechanically into a urine-collecting device allowing exact determination of the drained volume (manual technique). A detailed fluid balance was calculated every hour ( and ). If the desired volume of the dwell exceeded 60 mL, the installation of PD fluid was carried out using a Baxter HomeChoice PD system (automated technique). The dialysate solutions used were standard commercial preparations (Dianeal PD-2; Baxter International Inc.); heparin (500 U/L of dialysate) and potassium chloride were added taking into account the patient’s potassium level. The dextrose concentration varied from 1.36% to 3.86%, and the choice of dextrose concentration depended on the presence of hypervolemia and edema. Standard initial prescription consisted of 10 min for installation of fluid and 30–45 min dwell time followed by 15 min of drainage. If needed, to achieve adequate dialysis, the dwell volume was gradually increased and the cycles were individualized. Fluid overload was eliminated by diuretics in nonoliguric patients and/or the use of hypertonic PD fluids in anuric patients. Indications for discontinuation of PD treatment included a return to a sufficient urine output, maintaining a negative fluid balance, normalization of the serum electrolytes and acid–base status, as well as ammonium levels.
Figure 1. Set-up for PD treatment of an infant who needed a dwell volume of less than 60 mL. (A) Infusion pump; (B) PD fluid bag and PD machine for heating purposes; (C) three-way router; (D) patient; and (E) urine-collecting device for measurement of drained volume of PD fluid.
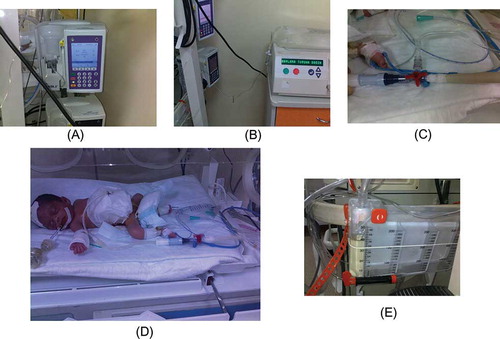
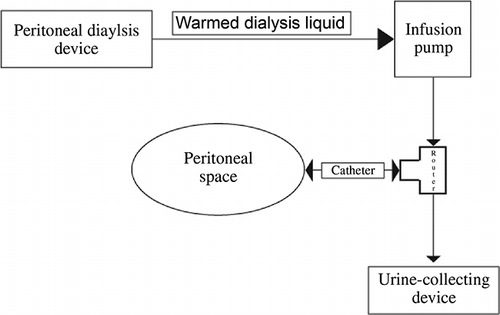
Figure 2. Manual PD technique (connection and liquid flow direction; set dwell volume and infusion time with infusion pump which takes warmed dialysis liquid from PD device. Pumping route is through into peritoneal space. Control filling and drainage with three-way router. While filling phase, close drainage side of the router. When filling phase is done, three-way router is setted completely closed for dwelling. After that, open drainage side for flowing out. Drained liquid is collected with urine-collecting device to measure ultrafiltration).
Statistics
We compared categorical data with the χ2-test. Student’s t-test was used to test equality of means of different groups. A p-value of less than 0.05 was considered as significant.
RESULTS
In these 27 neonates, the average gestational age and birth weight were 35.18 ± 4.02 weeks (min. 27 weeks, max. 41 weeks) and 2534.62 ± 897.41 g (min. 1000 g, max. 3800 g), respectively. The mean PD duration time was 6.11 ± 6.30 days (min. 1 day, max. 25 days). Of these, 10 patients were treated manually [mean birth weight: 1584 ± 528.60 g (min. 1000 g, max. 2500 g)], whereas 17 patients were treated with automated system [mean birth weight: 3093.82 ± 504.45 g (min. 2100 g, max. 3800 g)]. Pre- and post-dialysis blood creatinine, urea, sodium, and potassium levels of all patients are given in . PD-related complications were seen in seven patients (25.92%) (peritonitis in three patients, obstruction in three patients, and leakage in one patient). Peritonitis was treated by systemic and/or intraperitoneal antibiotics. Obstruction was eliminated by catheter revision in two patients. One patient who showed persistent obstruction required catheter replacement (patient no. 9). Leakage was managed by reduction in the dwell volume. Among 27 neonates, 16 patients died (8 out of 13 preterm babies, 8 out of 14 term neonates). Underlying reasons were metabolic disease (in six), asphyxia (in four), sepsis (in two), RDS (in one), PUV (in one), CoA (in one), and TAPVR (in one). Eleven patients (five preterm and six term neonates) were discharged from NICU. These 11 babies were on nephrologic follow-up.
Table 1. Demographic, biochemical, and PD features of the study group.
shows the comparison of patient characteristics between infants who survived and those who died. There was no statistically significant difference in risk factors evaluated between both groups.
Table 2. Comparison of patient characteristics between infants who survived and those who died.
DISCUSSION
In this retrospective study, we highlight that PD treatment could be applied in all neonates, even in highly risky infants. Of note, minor or major manipulations should be put into practice to accommodate low-birth-weight infants in PD.
PD treatment is relatively easy and technically simple and can be performed even in hemodynamically unstable patients.Citation1 Although PD can be applied in all NICUs, when necessary, technical manipulations should be put into practice to accommodate smaller infants undergoing dialysis. We are sure that all NICUs face some problems at every phase of acute PD treatment. At that point, centers must need to find out some special solutions to accommodate low-birth-weight newborns in acute PD dialysis treatment program. In this study, especially in smaller infants, who needed a dwell volume of <60 mL, the acute PD technique required some manipulations as the automated PD device works minimally at 60 mL of dwell volume. In our institution, we were not able to use an automated device working less than 60 mL due to unavailability. Therefore, we designed a simple, mechanical, low-tech, and closed PD system. This system consisted of an infusion pump (A), a PD device as heater (B), a three-way router (C), a peritoneal catheter (D), and a urine-collecting device (E). The principle of this manual PD system processing is described in detail in . This study is the first we know of to perform such a manual PD technique among low-birth-weight infants undergoing acute dialysis. However, its application is associated with some special problems. One person must be assigned responsibility to follow up that system strictly. A liquid leakage is another common problem, especially from connection parts of mechanical, low-tech, and closed PD system. The risk of infection is likely high. In addition, drained volume must be recorded on a PD chart per hour. Concerning these technical difficulties, we were able to perform manual PD in 10 smaller infants. Of these, four patients got benefited from manual PD system and they could be discharged. The risk of complications was comparable to those in automated PD.
In the present single-center retrospective study, we found that newborns requiring renal replacement treatment due to various reasons were associated with substantially increased mortality. In our series, 16 patients died and the overall mortality was found to be 59.25%. However, almost half of our study group (13 out of 27) consisted of preterm babies. This situation seems to play an important role in high mortality rate. Additionally, having major comorbid illnesses, listed in , might contribute to increased mortality rate. In agreement, the overall mortality rate of 59.25% in our study population is consistent with previous studies.Citation3 On the other hand, Cataldi et al.Citation4 and Csaicsich et al.Citation5 demonstrated higher mortality rates (80% and 69%, respectively) for preterm infants with renal failure, regardless of their weight or gestational age. In general, sick preterm infants are assumed to die from multiorgan failure rather than from renal failure.Citation3–5 Therefore, clinicians in many NICUs do not routinely wish to initiate dialysis in preterm infants with renal failure. However, in our series, 5 out of 13 preterm infants survived. We believe that, in fact, acute PD treatment can improve the outcome of these preterm infants. It is clear that data from larger registries and/or prospective multicenter, randomized, controlled studies will be required to evaluate the efficacy and safety of this intervention.
In experienced hands, major complications of PD are few. As expected, the time on PD is associated with an increased risk of PD-related complications. It is well known that, however, complication rates for infants on PD are also higher than those seen for older PD children. Neonates who receive acute PD have multiple risk factors for complications, including low body surface area, placement of the catheter, prematurity, and other comorbid diseases. Our results showed that the overall rate of PD-related complications in neonates is comparable to those previously reported studies.Citation1,2,6 Specifically, the rate was 25.92% (seven patients; obstruction in three, peritonitis in three, and leakage in one). In our series, we terminated PD due to catheter malfunction in one patient (patient no. 9). This patient required catheter replacement on two occasions due to persistent obstruction by omentum. This patient died 4 days after termination of PD treatment.
In a portion of the publications to date, experiences are especially associated with infants operated for congenital heart disease and receiving PD. Due to special causes such as complexity of the underlying heart disease and surgical procedure, duration of cardiopulmonary bypass, circulatory arrest, postoperative low cardiac output syndrome, and use of adrenaline and isoprenaline, it has been reported that the mortality rate in AKI newborns due to cardiac surgery is higher than that due to causes other than cardiac surgery.Citation7–10 To date, unfortunately, we were not able to report our experience with acute PD in neonates who needed cardiac surgery due to the lack of pediatric cardiac surgeon in our institution.
Children with urea cycle defects and organic acidemias often present with metabolic acidosis, hyperammonemia, and clinical symptoms of acute metabolic encephalopathy, including seizures, apnea, or coma, within the first days of life. The duration and extent of hyperammonemia predict the neurological outcome, making immediate removal of ammonia a critical issue.Citation11,12 The majority of our patients with metabolic disease undergoing PD died on days 1–25; one newborn had citrullinemia whereas the diagnosis of the other newborns remained unclear. In the past 10–15 years, technical developments made HD, especially continuous techniques, suitable for neonates by reducing the volume of the extracorporal circuit, having pumps that work with high accuracy, and by providing small-sized central venous catheters.Citation13 We are aware that continuous HD techniques can lead to even more pronounced advantages in overall survival in these patients. Therefore, in our institution, we should revise our treatment strategies in newborn patients with metabolic disease requiring dialysis.
In conclusion, PD application in neonates, especially in preterm babies, is associated with substantially increased mortality. It should be performed exclusively by a highly experienced team. Automated PD devices working at low dwell volume will not be available in each center. Thus, the lack of appropriate devices requires that each center should create its own solutions on PD treatment, especially, in low-birth-weight infants. However, further studies and/or more registry data are needed to determine whether these infants die from other causes rather than from AKI or dialysis would improve the outcome in this special population.
ACKNOWLEDGMENT
We thank Assoc. Prof. Esra Arun Ozer, MD, Director of NICU, Izmir Tepecik Training and Research Hospital, and her colleagues for the meticulous caring for these newborn infants.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Yu JE, Parl MS, Pai KS. Acute peritoneal dialysis in very low birth weight neonates using vascular catheter. Pediatr Nephrol. 2010;25:367–371.
- Aksu N, Yavascan O, Anil M, Kara OD, Erdogan H, Bal A. A ten-year single-center experience in children on chronic peritoneal dialysis, significance of percutaneous placement of peritoneal dialysis catheters. Nephrol Dial Transplant. 2007;22: 2045–2051.
- Hentschel R, Lodige B, Bulla M. Renal insufficiency in the neonatal period. Clin Nephrol. 1996;46:54–58.
- Cataldi L, Leone R, Moretti U, . Potential risk factors for the development of acute renal failure in preterm newborn infants: A case-control study. Arch Dis Child Fetal Neonatal. 2005;90: 514–519.
- Csaicsich D, Russo-Schlaff N, Messerschmidt A, Weninger M, Pollak A, Aufricht C. Renal failure, comorbidity and mortality in preterm infants. Wien Klin Wochenschr. 2008;120:153–157.
- Pedersen KR, Hjortdal VE, Christensen S, . Clinical outcome in children with acute renal failure treated with peritoneal dialysis after surgery for congenital heart disease. Kidney Int. 2008;7(3):81–86.
- Chien JC, Hwang BT, Weng ZC, Meng LCC, Lee PC. Peritoneal dialysis in infants and children after open heart surgery. Pediatr Nephrol. 2009;50:275–279.
- Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;28:877–881.
- Larsen SH, Pedersen J, Jacobsen J, Johnsen SP, Hansen OK. Hjortdal V. The RACHS-1 risk categories reflect mortality and length of stay in a Danish population of children operated for congenital heart disease. Eur J Cardiothorac Surg. 2005;45: 407–413.
- Pedersen KR, Povlsen JV, Christensen S, . Risk factors for acute renal failure after surgery for congenital heart disease in children. Acta Anaesthesiol Scand. 2007;51:1344–1349.
- Burton BK. Inborn errors of metabolism in infancy: A guide to diagnosis. Pediatrics. 1998;102:e69.
- Bachmann C. Hyperammonämien. In: Lentze MJ, Schulte FJ, Schaub J, Spranger J, eds. Pädiatrie. 3rd ed., Berlin, Heidelberg: Springer; 2007:329–334.
- Arbeiter AK, Kranz B, Wingen AM, . Continuous venovenous hemodialysis (CVVHD) and continuous peritoneal dialysis (CPD) in the acute management of 21 children with inborn errors of metabolism. Nephrol Dial Transplant. 2010;25: 1257–1265.
