Abstract
Background/Aims: Ghrelin plays a central role in the regulation of gastrointestinal (GI) motility. This study aimed to investigate the expression of ghrelin and growth hormone secretagogue receptor (GHSR) in the central nervous system of rats with chronic renal failure (CRF). Methods: Sprague-Dawley rats (male, 180 ± 20 g, n = 24) were treated by 5/6 nephrectomy to construct CRF model. As their plasma creatinine concentration and blood urea nitrogen were maintained more than double the normal level for 2 weeks, they were killed for assessing the expression of ghrelin and GHSR in hypothalamus and hippocampus using immunohistochemistry and real-time polymerase chain reaction (RT-PCR). The rats (male, 180 ± 20 g, n = 24) treated by Sham operation served as a control. One-way analysis of variance and Student–Newman–Keuls q test were used to analyze group difference and a p-value of <0.05 was considered as statistically significant. Results: Compared with the controls, the ghrelin and GHSR expression was obviously increased in the hippocampus (p < 0.05) but decreased in the hypothalamus of rats with CRF (p < 0.05). Conclusions: CRF was found to impact the expression of ghrelin and GHSR in hypothalamus and hippocampus. This might be associated with the CRF-induced GI motility dysfunction.
INTRODUCTION
Patients with chronic renal failure (CRF) are often complicated by gastrointestinal (GI) motor dysfunction.Citation1 Reportedly, those patients receiving conservative treatment or peritoneal dialysis often suffer from the obviously delayed gastric emptying,Citation2 the decreased small intestinal motility,Citation3 and the frequent functional bowel disorders, such as functional vomiting and irritable bowel syndrome.Citation4 Even before the pathological injury appearing in the upper GI tract, the GI motor disorder may have some abnormalities in migrating motor complex (MMC) at duodenum and jejunum and in colonic transit time.Citation5 Our prior research on gastric emptying, small intestinal transit, interdigestive myoelectric complex (IMC), and fecal water content in the rats with CRF constructed by 5/6 nephrectomy (5/6 NX) proved that the GI motility was impaired by CRF.Citation6 How and why this situation happens aroused our great interest.
GI motility is regulated partly by autonomic nervous system and partly by central nervous system (CNS). It has been confirmed that hypothalamus and hippocampus are involved in the regulation of GI motility.Citation7 The hypothalamus is one of the two main channels for the brain to inform itself of the metabolic status of an individual. Hormonal signals reflecting the availability and demand for metabolic fuel are relayed via the neurons located in the arcuate nucleus (Arc) in the mediobasal hypothalamus, alongside the third ventricle.Citation8 The hippocampus is an integrative relay station of the limbic system that is reciprocally connected with the amygdala, the hypothalamus, and the medulla oblongata.Citation9 Besides recognition function, for example, learning, memory, and emotion, hippocampus also plays a role in the regulation of feeding behavior, satiety, and GI motility of mammal.Citation10
Ghrelin, a 28-amino-acid brain gut peptide, is mainly produced by the X/A-like endocrine cells of the gastric oxyntic mucosa and partly by the hypothalamic arcuate nucleus, pituitary, kidney, and placenta.Citation11,12 Ghrelin has multiple functions, including the regulation of growth hormone secretion and energy balance, the improvement of cell proliferation and the protection of neurons,Citation13 and the regulation of GI motility via the CNS.Citation14 Ghrelin plays its role through activating the functionally active, signal-transducing form of growth hormone secretagogue receptor (GHSR), a G protein-coupled receptor.Citation15 In the CNS, ghrelin and GHSR are mainly distributed in pituitary, hypothalamic arcuate nucleus, ventromedial nucleus (VMN), and hippocampus.Citation16,17
Previous studies have proven that hypothalamic administration of ghrelin can improve the feeding, colonic propulsion, and IMC or MMC of ratsCitation18,19 and that hippocampal microinjection of ghrelin and/or motilin can increase food intake Citation20 and amplitude of gastric contractions.Citation10,21 Guan et al.Citation10 suggested the two regions were the important components to construct the pathway, a limbic (hippocampus)–hypothalamus–brain stem–vagus pathway, through which motilin and/or ghrelin regulates GI motility via CNS.
Recently, Ogiso et al.Citation22 argued that ghrelin might be associated with functional dyspepsia through its effect on the regulation of gut motility. Getting inspired from this, we hypothesized that there would be a link between ghrelin and the CRF-induced GI motor dysfunction. Thereupon, we performed this study to investigate whether CRF would produce an effect on the expression of ghrelin and its receptor GHSR in the hypothalamus and hippocampus. We found that the ghrelin and GHSR expression was obviously altered in the hypothalamus and hippocampus of rats with CRF, by which we hope to lay the foundation for further studies on the possible pathogenesis of CRF-induced GI motor dysfunction.
MATERIALS AND METHODS
Ethics Statement and Animals
Animal use was approved by the Ethics Committee of the Second Affiliated Hospital of Medical School of Xi’an Jiaotong University (approval ID: 2010-LS-0106). Sprague-Dawley rats (male, 180 ± 10 g) were purchased from Laboratory Animal Center in the Fourth Military Medical University and randomly classified into CRF group and control group (n = 24 per group). At the beginning of the experiment, the rats were about 7–8 weeks old. They were housed under standardized conditions in plastic cages (light–dark cycle 12/12 h, temperature 22 ± 2°C, humidity 50 ± 10%), fed with standard diet (provided by the Laboratory Animal Center in Medical School of Xi’an Jiaotong University), and had free access to tap water. Animal care and treatment were conducted in conformity with institutional guidelines that are in compliance with international laws and politics.
CRF Model by 5/6 NX
This study used 5/6 NX to construct CRF model and Sham operation to treat the control rats. The 5/6 NX and Sham operation were performed as described elsewhere.Citation6 After surgery, all the rats were fed with normal diet in single cage and administrated with 3 days of penicillin intraperitoneal injection (200,000 U per day). From the rat tail vein, 0.5 mL blood was sampled every 2 weeks for the detection of plasma creatinine (PCr) and blood urea nitrogen (BUN) using automatic biochemical analyzer (7170A, Hitachi Co., Ltd., Tokyo, Japan).
Immunohistochemical (IHC) Analysis
When the PCr and BUN values of CRF group were maintained more than double the normal level for 2 weeks, we started the immunohistochemical (IHC) analysis. The rats were fixed on an operating table in a supine position after they were anesthetized by intraperitoneal injection of 10% chloral hydrate (3 mL/mg). The thoracic cavities of rats were opened to expose the pericardium, right ventricle, and right atrial appendage. Eighty milliliters of physiological saline (80 mL) and 400 mL of 4% paraformaldehyde buffer were rapidly injected into the left ventricle and ascending aorta. Immediately thereafter, the rat brain was removed and followed by the next steps one after another: tissue fixation (8 h in 4% paraformaldehyde buffer), rinsing tissue with purified water, dehydration through graded ethanol solutions, rapid freezing in liquid nitrogen, embedding tissues in paraffin blocks, serial section cutting (thickness 10 μm), and then pasting the sections on poly-lysine-coated slides.
StreptAvidin–Biotin Complex (SABC) IHC staining was employed. The primary antibodies of ghrelin and GHSR were rabbit anti-ghrelin (rat) antibody (1:2500) and rabbit anti-GHSR (human) antibody (1:100), respectively. The secondary antibody of ghrelin and GHSR was biotinylated goat anti-rabbit IgG (1:1000–3000). All antibodies were purchased from Phoenix Biotech Co., Ltd. (Beijing, China).
The identification of hypothalamus and hippocampus in rat brain was performed according to the Atlas of 73 Transverse Levels in Structure of the Rat Brain (Third Edition) by Larry Swanson.Citation23 As depicted by A, we observed the expression of ghrelin and GHSR [Diaminobenzidine (DAB) coloration, magnification 400×] and measured the gray values of 10 different high-power fields (HPFs) that were randomly selected within the hypothalamus or hippocampus using ImageJ. The protein expression level of ghrelin and GHSR in the hypothalamus or hippocampus was assessed by the mean value of the 10 HPFs.
Figure 1. (A) The hippocampus (1) and hypothalamus (2) are identified on the coronal section of rat brain (DAB coloration) according to the Rat Brain Structure by Larry Swanson. The ghrelin protein expression (B) and the GHSR protein expression (C) in the hippocampus and hypothalamus of both groups are showed with a 400-fold magnification. The gray mean ± SD of ghrelin (D) and GHSR (E) in the two regions demonstrate the between-group differences in protein expression (n = 16 per subgroup, including eight controls and eight CRF rats). The OD mean ± SD of ghrelin (F) and GHSR (G) show the difference in mRNA expression between the two groups (n = 16 per subgroup, including eight controls and eight CRF rats).Note: *p < 0.05 and **p < 0.01.
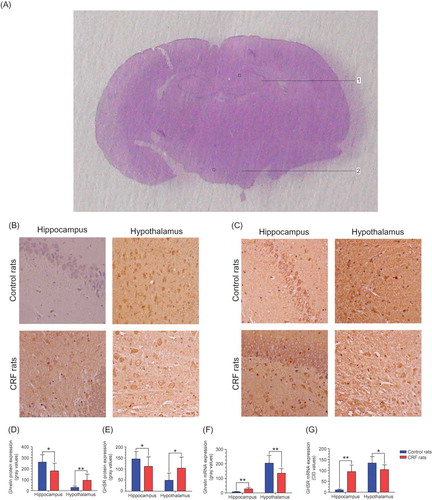
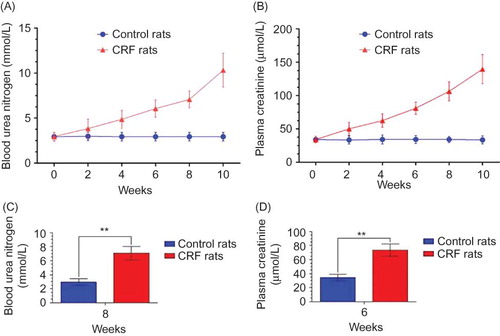
Figure 2. The BUN (A) and PCr (B) of both groups (n = 10 per group) are documented every 2 weeks for observing their dynamic changes to determine when to start the detection of ghrelin and GHSR expression. The BUN value (C) of CRF rats in the 8th week and the PCr value (D) in the 6th week were more than double the normal value. By the 10th week, the two values were maintained at a higher level for or over 2 weeks, indicating that we could start the next experiment.Note: **p < 0.01.
Isolation and Determination of RNA
Tissue samples (0.1 g) of the hypothalamus and hippocampus were collected after the identification of the two regions. RNA isolation was performed using Trizol Kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s instructions. After reverse transcription, the resulting materials were used for polymerase chain reaction (PCR) amplification using gene-specific primer pairs and SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Ghrelin and GHSR mRNA were determined quantitatively by using Bio-Rad IcycLer iQ™ (Bio-Rad, Hercules, CA, USA) and β-actin as intra-contrast gene or internal control.
The sequences of β-actin primer were as follows: upper primer: 5′-TCC TAG CAC CAT GAA GAT C-3′ and lower primer: 5′-AAA CGC AGC TCA GTA ACA G-3′. The sequences of ghrelin primer were as follows: upper primer: 5′-GAA AGG AAT CCA AGA AGC CA-3′ and lower primer: Reverse 3′-GGA GCA TTG AAC CTG ATT TC-5′. The sequences of GHSR primer were as follows: upper primer: 5′-CGA CCT GCT CTA GCA AAC TC-3′ and lower primer: 3′-CAC GCC CAC CAG CAC GAA GA-5′.
For real-time PCR (RT-PCR), the amplification conditions of ghrelin were initial denaturation (95°C, 3 min), 40 cycles of denaturation (95°C, 10 s), annealing (60.5°C, 10 s), extension (72°C, 10 s), and then a final extension (72°C, 10 min). And the amplification conditions of GHSR were initial denaturation (95°C, 3 min), 40 cycles of denaturation (95°C, 10 s), annealing (63°C, 10 s), extension (72°C, 10 s), and then a final extension (72°C, 10 min).
Statistics
All data were expressed as mean ± SD. Group differences were analyzed using one-way analysis of variance and pairwise comparison using Student–Newman–Keuls q test. A p-value of <0.05 was considered as statistically significant.
RESULTS
Assessment of CRF Model
Before surgery all the rats were healthy, and there were no difference in BUN and PCr between the two groups. Since the surgery, the BUN (A) and PCr (B) of CRF rats became higher and higher gradually, but those of controls had no obvious alteration. The BUN (C) and PCr values (D) of CRF rats were over double the normal level, respectively, in the 8th week and 6th week and kept ascending in the 10th week. These results indicated that our construction of CRF model was successful.
Protein and Gene Expression
The biweekly lab examinations confirmed that by the 10th week the high levels (more than double the normal level) of PCr and BUN in CRF rats were maintained over 2 weeks. Subsequently, we conducted the detection of ghrelin and GHSR expression.
On the IHC stained sections, it was observed that ghrelin protein (B) presented with the yellow-brown granules in the membrane and/or cytoplasm of ghrelin-positive cells, and the GHSR protein (C) was also stained yellow or brown and was only located in the membrane of GHSR-positive cells. The ghrelin expression was not detected in the hippocampus of control rats. In contrast, the hippocampus of CRF rats had ghrelin expression. Compared with the controls, the mean gray value of ghrelin in the hippocampus of CRF rats was obviously elevated (p < 0.05) but that in the hypothalamus was decreased (p < 0.05) (D). Similarly, the mean gray value of GHSR was increased in the hippocampus (p < 0.05) but decreased in the hypothalamus of the CRF rats (p < 0.05) (E).
Bio-Rad IcycLer iQ™ was used to read the optical density (OD) at 260 and 280 nm. Each sample was tested in triplicate, and the ratio of OD260/OD280 nm of each sample ranged from 1.8 to 2.0. Compared with the controls, the OD value of ghrelin mRNA expression was increased in the hippocampus of CRF rats (p < 0.01) but decreased in the hypothalamus (p < 0.01) (F), likewise for the GHSR mRNA expression (G). In summary, the findings of IHC and RT-PCR demonstrated that the ghrelin and GHSR expression was upregulated in the hippocampus and downregulated in the hypothalamus of CRF rats.
DISCUSSION
We would like to emphasize that our findings were observed under the condition that the PCr and BUN values in CRF rats were maintained more than double the normal value for 2 weeks or more. It is important because the expression alteration of ghrelin and GHSR in the CNS of CRF would not be detected if the uremic effect is too weak or lasts too short. These findings, we think, may confirm that CRF produces an effect on the expression level of ghrelin and GHSR in the CNS.
Although the ghrelin content of brain is very low,Citation11 it has been demonstrated that ghrelin mainly exists in the hypothalamic regions because the ghrelin receptor GHSR is mainly expressed in the hypothalamus and pituitary.Citation16,24 Outside the hypothalamus, ghrelin is also observed in pyramidal neurons of layer V in the sensorimotor area, in the cingulate gyrus of the cerebral cortex, and in the dorsal vagal complex of the medulla oblongata,Citation25 and GHSR in the dentate gyrus, CA2 and CA3 regions of the hippocampus, thalamic regions, and several nuclei within the brain stem.Citation24,26 In IHC stained sections, GHSR presents with the yellow-brown stained granules and is only distributed in the membrane of positive cells.Citation27 Generally, our results are in line with these previous studies except the detection of ghrelin expression in the hippocampus of CRF rats.
The main site of ghrelin synthesis in the CNS is hypothalamus, including the lateral hypothalamus, the arcuate nucleus (Arc), the VMN, the dorsomedial nucleus, the paraventricular nucleus, and the ependymal layer of the third ventricle.Citation27 GHSR type 1a is highly expressed in the Arc and VMN.Citation24,28 This may be the reason why the decreased production of brain-gut peptides in the hypothalamus results in the reduction of GI motility.Citation29 Our previous research has demonstrated that the GI motility of rats with CRF is reduced.Citation6 This study’s results proved that the expression of ghrelin and GHSR was obviously decreased in the hypothalamus of CRF rats. These evidences can indirectly suggest that there might be a close relationship between the GI motility dysfunction and the altered expression of ghrelin and GHSR in the hypothalamus of CRF rats.
Under normal circumstances, GHSR can be detected in hippocampus but not ghrelin.Citation24–27 Our results proved this as well. Interestingly, this study found that the CRF rats had ghrelin expression in the hippocampus and had a higher expression of GHSR than the control rats in the hippocampus. The latter means that the hippocampus of CRF rats could have an enhanced sensitivity to ghrelin. Carlini et al.Citation20 indicated in 2004 that hippocampus was the structure most implicated in memory retention induced by ghrelin. Thus, we think that this finding may be more related to the possible processes of learning and memory of hippocampus. In addition, we surmise that this finding might also be related to early satiety, a frequent symptom in patients with CRF,Citation30 because satiety is one of multiple important functions of the hippocampus.Citation10 Certainly, this speculation needs further verification.
The production of growth hormone within the hippocampus needs to respond to age, sex, and stress.Citation31 Therefore, this study only selected male rats with same week age and provided them with the same standardized housing and feeding conditions, by which we precluded adverse influences from age, sex, and stress. But, we did not detect the expression of ghrelin and GHSR in nucleus tractus solitarius (nTS), which might be a limitation of this study. Because nTS is also an important channel for the brain to get the visceroceptive information carried by vagus nerve to regulate the metabolic status.Citation8 Another limitation of this study ought to be that since we did not perform correlation analysis, we lack direct evidence to show the relationship between alterations of the ghrelin system in the CNS and reduced GI motility in CRF rats.
The main source of ghrelin acting on central neurons seems to be from the peptide synthesized in the stomach and released into the general circulation.Citation32,33 Would CRF impact the ghrelin system in GI tract and/or the serum ghrelin level? What are the factor(s) that alter the ghrelin system in the brain? Is it increased PCr or BUN? Are there other possible factors that induce GI motor dysfunction in CRF, such as decreased food intake or negative energy balance? What is happening in CRF? All of these questions need more explorations. Yet, in spite of that, our results can confirm that CRF produces an effect on the expression of ghrelin and GHSR in the hypothalamus and hippocampus. It might be associated with the CRF-induced GI motor dysfunction.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by grants from the National Natural Science Foundation of China (No. 81070590), the Key Projects of the Clinical Science by Chinese Ministry of Health (No. 06-9602-13), and the Guide Projects for Science and Technology Development by Xi’an Science and Technology Bureau (No. SF08002).
REFERENCES
- Hirako M, Kamiya T, Misu N, . Impaired gastric motility and its relationship to gastrointestinal symptoms in patients with chronic renal failure. J Gastroenterol. 2005;40(12):1116–1122.
- Strid H, Simren M, Stotzer PO, Abrahamsson H, Bjornsson ES. Delay in gastric emptying in patients with chronic renal failure. Scand J Gastroenterol. 2004;39(6):516–520.
- Strid H, Simren M, Stotzer PO, Ringstrom G, Abrahamsson H, Bjornsson ES. Patients with chronic renal failure have abnormal small intestinal motility and a high prevalence of small intestinal bacterial overgrowth. Digestion. 2003;67(3):129–137.
- Kahvecioglu S, Akdag I, Kiyici M, . High prevalence of irritable bowel syndrome and upper gastrointestinal symptoms in patients with chronic renal failure. J Nephrol. 2005;18(1):61–66.
- Lefebvre HP, Ferre JP, Watson AD, . Small bowel motility and colonic transit are altered in dogs with moderate renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R230–R238.
- Fu RG, Wang Y, Yuan HZ, . Effects of chronic renal failure on gastrointestinal motility: A study on the changes of gastric emptying, small intestinal transit, interdigestive myoelectric complex, and fecal water content. Ren Fail. 2011;33(6):615–621.
- Broberger C. Brain regulation of food intake and appetite: Molecules and networks. J Intern Med. 2005;258(4):301–327.
- Broberger C, Hokfelt T. Hypothalamic and vagal neuropeptide circuitries regulating food intake. Physiol Behav. 2001; 74(4–5):669–682.
- Seifert W. Neurobiology of the Hippocampus. New York: Academic Press; 1983.
- Guan Y, Tang M, Jiang Z, Peeters TL. Excitatory effects of motilin in the hippocampus on gastric motility in rats. Brain Res. 2003;984(1–2):33–41.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660.
- Inhoff T, Monnikes H, Noetzel S, . Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29(12):2159–2168.
- Xu J, Wang S, Lin Y, Cao L, Wang R, Chi Z. Ghrelin protects against cell death of hippocampal neurons in pilocarpine-induced seizures in rats. Neurosci Lett. 2009;453(1):58–61.
- Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol Rev. 2009;61(4):430–481.
- Gnanapavan S, Kola B, Bustin SA, . The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87(6):2988.
- Howard AD, Feighner SD, Cully DF, . A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977.
- Nakazato M, Murakami N, Date Y, . A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198.
- Tebbe JJ, Mronga S, Tebbe CG, Ortmann E, Arnold R, Schafer MK. Ghrelin-induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1- and corticotrophin-releasing factor 1 receptor activation. J Neuroendocrinol. 2005;17(9):570–576.
- Olszewski PK, Grace MK, Billington CJ, Levine AS. Hypothalamic paraventricular injections of ghrelin: Effect on feeding and c-Fos immunoreactivity. Peptides. 2003;24(6): 919–923.
- Carlini VP, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313(3):635–641.
- Peeters TL. Central and peripheral mechanisms by which ghrelin regulates gut motility. J Physiol Pharmacol. 2003;54(Suppl. 4):95–103.
- Ogiso K, Asakawa A, Amitani H, Inui A. Ghrelin: A gut hormonal basis of motility regulation and functional dyspepsia. J Gastroenterol Hepatol. 2011;26(Suppl. 3):67–72.
- Swanson LW. Brain Maps III: Structure of the Rat Brain: An Atlas with Printed and Electronic Templates for Data, Models, and Schematics. 3rd rev. ed. Boston, MA: Elsevier, Academic Press; 2004.
- Guan XM, Yu H, Palyha OC, . Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48(1):23–29.
- Hou Z, Miao Y, Gao L, Pan H, Zhu S. Ghrelin-containing neuron in cerebral cortex and hypothalamus linked with the DVC of brainstem in rat. Regul Pept. 2006;134(2–3):126–131.
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494(3):528–548.
- Cowley MA, Smith RG, Diano S, . The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661.
- Bennett PA, Thomas GB, Howard AD, . Hypothalamic growth hormone secretagogue-receptor (GHS-R) expression is regulated by growth hormone in the rat. Endocrinology. 1997; 138(11):4552–4557.
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001; 50(11):2438–2443.
- Strid H, Simren M, Johansson AC, Svedlund J, Samuelsson O, Bjornsson ES. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol Dial Transplant. 2002;17(8):1434–1439.
- Donahue CP, Kosik KS, Shors TJ. Growth hormone is produced within the hippocampus where it responds to age, sex, and stress. Proc Natl Acad Sci USA. 2006;103(15):6031–6036.
- Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Curr Neuropharmacol. 2009;7(1):37–49.
- Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85(2):495–522.
