Abstract
The results from the published studies on the relationship between GSTM1/GSTT1 null genotypes and renal cell carcinoma (RCC) risk are still conflicting. This meta-analysis was performed to evaluate the relationship between GSTM1/GSTT1 null genotypes and RCC susceptibility. Association studies were identified from the databases of PubMed, Embase, Cochrane Library, and CBM-disc (China Biological Medicine Database) on 1 February 2012, and eligible investigations from 1950 to 2012 were synthesized using meta-analysis method. Results were expressed as odds ratios (ORs) for dichotomous data, and 95% confidence intervals (CIs) were also calculated. Six studies were identified for the analysis of association between polymorphic deletion of GSTM1/GSTT1 and RCC risk. There was no association between GSTM1/GSTT1 null genotype and RCC susceptibility (GSTM1: N = 6, p-heterogeneity = 0.07, OR = 1.07, 95% CI: 0.85–1.35, p = 0.57; GSTT1: N = 6, p-heterogeneity < 0.00001, OR = 0.98, 95% CI: 0.58–1.65, p = 0.94). Interestingly, null genotype of GSTT1 was associated with RCC risk in Caucasians and Asians (Caucasians: N = 4, p-heterogeneity = 0.38, OR = 0.76, 95% CI: 0.61–0.95, p = 0.01; Asians: N = 1, OR = 2.39, 95% CI: 1.63–3.51, p < 0.00001). For the GSTM1–GSTT1 interaction analysis, the dual null genotype of GSTM1/GSTT1 was not significantly associated with RCC susceptibility (N = 4, p-heterogeneity = 0.006, OR = 1.17, 95% CI: 0.98–1.39, p = 0.09). However, the dual null genotype of GSTM1–GSTT1 was associated with RCC risk in Asians (N = 1, OR = 2.06, 95% CI: 1.36–3.13, p = 0.007). In conclusion, our study results suggest that GSTT1 null genotype is associated with the RCC susceptibility in Caucasians and Asians, and the dual null genotype of GSTM1–GSTT1 is associated with RCC risk in Asians. However, more genetic epidemiological investigations are required to further explore this relationship.
INTRODUCTION
Renal cell carcinoma (RCC) is one of the most lethal urologic cancers and is highly resistant to both radiotherapy and chemotherapy.Citation1 Its survival rates are very low since most of patients develop metastases beyond the kidney at the time of diagnosis.Citation2 Early diagnosis for RCC is very difficult, and the etiology of RCC is unclear. Current evidence indicates that the gene polymorphism is associated with the RCC susceptibility.Citation3–5
Glutathione S-transferases (GSTs) are phase II enzymes that facilitate the detoxification of xenobiotics and also play important roles in antioxidant defense.Citation6,7 Genetic polymorphisms in GST genes might influence the detoxification activities of the enzymes predisposing individuals to cancer risk.Citation8 GSTM1 and GSTT1 gene deletions are the most commonly studied GST variants.Citation9 There were some investigations reporting that GSTM1 and GSTT1 gene deletions were associated with cancer risk.Citation10–12
Gene polymorphism has been reported to be an important factor that increases the risk of RCC. Phase II enzymes such as GSTM1 and GSTT1 play important roles in protecting cells against damage induced by carcinogens.Citation13 In the past decades, most of the epidemiological studies investigating the association of null genotype of GSTM1/GSTT1 with RCC susceptibility were conducted. However, the available evidences are weak at present, due to the sparseness of data or disagreements among the reported studies. The evidence from meta-analysis might be powerful compared with the individual investigation. This meta-analysis was performed to investigate whether null genotype of GSTM1/GSTT1 was associated with the onset of RCC, by widely collecting the reported investigations.
MATERIALS AND METHODS
Search Strategy for the Association of Null Genotype of GSTM1/GSTT1 with RCC Susceptibility
The relevant studies were searched from the electronic databases of PubMed, Embase, Cochrane Library, and CBM-disc (China Biological Medicine Database) on 1 February 2012, and eligible original articles published from 1950 to 2012 were selected for our study. The retrieval strategy of ‘glutathione S-transferases or GSTs or GSTM1 or GSTT1’ and ‘renal cell carcinoma or renal cancer or RCC’ was entered into the above-mentioned databases for search. The searches in PubMed and Embase were limited to human. Additional articles were identified through references cited in retrieved articles.
Inclusion and Exclusion Criteria
Inclusion Criteria
The inclusion criteria are as follows:
| 1. | The outcome had to be RCC. | ||||
| 2. | There had to be at least two comparison groups (RCC group vs. control group). | ||||
| 3. | Investigation should provide the data of GSTM1/GSTT1 genotype distribution. | ||||
Exclusion Criteria
The exclusion criteria are as follows:
| 1. | Review articles and editorials | ||||
| 2. | Case reports | ||||
| 3. | Preliminary result not on GSTM1/GSTT1 or outcome | ||||
| 4. | Investigating the role of GST gene expression in disease | ||||
| 5. | If multiple publications from the same study group occurred, we selected only the most complete paper for our final analysis. | ||||
Data Extraction and Synthesis
Two investigators independently extracted the following information from each eligible study: first author’s surname, year of publication, and the number of cases and controls for GSTM1/GSTT1 genotypes. Frequencies of null genotype of GSTM1 and GSTT1 were calculated for the case and the control groups, from the corresponding genotype distribution. The results were compared and the disagreement was resolved by discussion.
Statistical Analysis
Cochrane Review Manager Version 5 (Cochrane Library, Oxford, UK) was used to calculate the available data from each investigation. The pooled statistic was counted using the fixed effects model, but a random effects model was conducted when the p-value of heterogeneity test was 0.1.Citation14,15 Results were expressed as odds ratios (ORs) for dichotomous data, and 95% confidence intervals (CIs) were also calculated.Citation16,17 A p-value <0.05 was required for the pooled OR to be statistically significant.Citation18,19 I2 was used to test the heterogeneity among the included studies.Citation20–23 The Begg adjusted rank correlation testCitation24 and the Egger regression asymmetry testCitation25 were used for exploring publication bias (p < 0.1 was considered significant), when the number of the included studies was more than six.
RESULTS
Study Characteristics
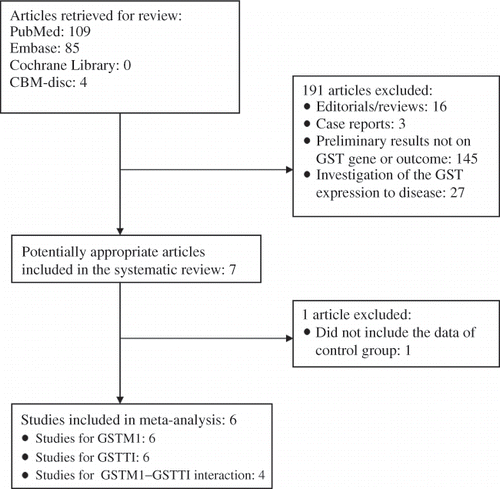
The studies reporting the relationship between GST gene polymorphism and RCC risk were identified from the databases, and six articlesCitation26–31 were selected for this meta-analysis ().
Study Characteristics for GSTM1 Null Genotype with RCC Risk
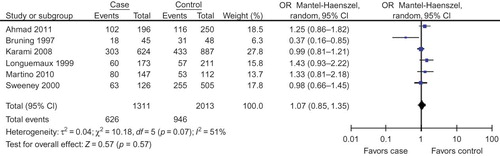
Six studiesCitation26–31 were selected for our investigation to study the relationship between GSTM1 null genotype and RCC susceptibility (). The data of our interest were extracted: first author’s surname, year of publication, and the number of cases and controls for GSTM1 genotype (). Those six investigations contained 1311 case series and 2013 controls. The average distribution frequency of GSTM1 null genotype in RCC cases was 46.62% and the average frequency in controls was 47.46%. The average distribution frequency of GSTM1 null genotype in cases was similar to that in controls (RCC/control = 0.98).
Table 1. Characteristics of the studies evaluating the effects of null genotypes of GSTM1 and GSTT1 on RCC risk.
Study Characteristics for GSTT1 Null Genotype with RCC Risk
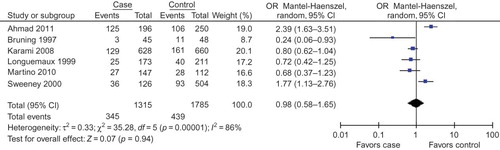
Six studiesCitation26–31 were selected for our investigation to study the relationship between GSTT1 null genotype and RCC risk (). The average distribution frequency of GSTM1 null genotype in RCC cases was 25.40% and the average frequency in controls was 25.42%. The average distribution frequency of GSTT1 null genotype in cases was similar to that in controls (RCC/control = 1.00).
Study Characteristics for Dual Null Genotype of GSTM1–GSTT1 with RCC Risk
Four studiesCitation26,27,29,31 were selected for our investigation to study the relationship between dual null genotype of GSTM1–GSTT1 and RCC susceptibility. The average distribution frequency of the dual null genotype of GSTM1–GSTT1 in RCC cases was 27.55% and the average frequency in controls was 25.46%. The average distribution frequency of the dual null genotype of GSTM1–GSTT1 in cases was slightly increased compared to that in controls (RCC/control = 1.08).
Association of GSTM1 Null Genotype with RCC Risk
In this meta-analysis, we found that GSTM1 null genotype was not associated with RCC risk in overall populations, Caucasians, and Asians ( for overall populations; ).
Table 2. Meta-analysis of the association of null genotypes of GSTM1 and GSTT1 with RCC risk.
Association of GSTT1 Null Genotype with RCC Risk
In this meta-analysis, we found that GSTT1 null genotype was not associated with RCC risk in overall populations ( for overall populations; ). Subgroup analysis according to different races was also performed, and we found that the GSTT1 null genotype was associated with the onset of RCC in Caucasians and Asians ().
Association of Dual Null Genotype of GSTM1–GSTT1 with RCC Risk
No association of the dual null genotype of GSTM1–GSTT1 with RCC risk was observed in overall populations (). Subgroup analysis according to different races was also performed, and we found that the GSTT1 null genotype was associated with the onset of RCC in Asians, but the association was not observed in Caucasians ().
Evaluation of Publication Bias
Publication bias test for the association of GSTM1 null genotype or GSTT1 null genotype with RCC risk in overall populations was performed. There were no publication bias for the association of GSTM1 null genotype or GSTT1 null genotype with RCC risk in overall populations (GSTM1: Begg p = 1.000, Egger p = 0.926; GSTT1: Begg p = 0.452, Egger p = 0.475).
DISCUSSION
The etiology of RCC was not elucidated and the genetic origin of RCC had been a focus of research in the past decades, and some investigations found that the genetic alteration could become an early diagnosis indicator to predict the onset of some cancers.Citation32–34 GSTs are implicated in the inactivation of procarcinogens that contribute to cancer progression.Citation35 There were a lot of significant evidences showing that the GSTs had taken part in the onset of RCC, and some studies found that some mutant sites of the GST genes might affect the activation of GSTs to play the multifunctional physiological processes. However, findings on the association of GSTM1/GSTT1 null genotype with RCC susceptibility have been controversial since the first investigation was reported. Most of the studies were performed in Caucasians. In this meta-analysis, we investigated whether the GSTM1/GSTT1 null genotype could become a valuable indicator to predict the risk of RCC and tried to draw a more robust conclusion.
In the analysis of null genotype of GSTM1 with RCC risk, our results indicated that GSTM1 null genotype did not predict the risk of RCC in overall populations, Caucasians, and Asians. Zhang et al.Citation36 performed a meta-analysis for the relationship of GSTT1 null genotype and oral cancer, and the results were not supportive for the association of GSTT1 null genotype with oral cancer risk. Chen et al.Citation37 conducted a meta-analysis to investigate the association between GSTT1 null genotype and gastric cancer risk, and the results were not supportive for the association of GSTT1 gene polymorphism with gastric cancer risk among Europeans, Americans, and East Asians. GSTM1 null genotype might not be associated with the onset of RCC. However, more studies should be performed in the future.
For the analysis of GSTT1 null genotype with RCC risk in Asians, our results indicated that GSTT1 null genotype could predict the susceptibility of RCC. In this study, we found that the GSTT1 null genotype was not associated with RCC susceptibility in overall populations, but the association was observed in Caucasians and Asians. Gao et al.Citation38 conducted a meta-analysis and reported that the null genotype of GSTT1 was associated with a significantly increased risk of cervical neoplasia in Asians. Some other investigationsCitation39,40 also found that GSTT1 gene polymorphism was a risk factor for cancer in Asians using meta-analysis method. Liao et al.Citation41 conducted a meta-analysis and found that null genotype of GSTT1 was associated with the risk of colorectal cancer, especially in Caucasian and Asian population. GSTT1 null genotype might be a risk factor for cancer morbidity in Caucasians and Asians.
Among the included studies, the study of De Martino et al.Citation30 did not provide the detailed genotype distributions for GSTM1 and GSTT1 for the control group, and we calculated the null genotype of the controls using the average distribution frequency of GSTM1/GSTT1 null genotype. Furthermore, we also excluded the study of De Martino et al.Citation30 for meta-analysis and found that the results were similar to those in the meta-analysis including the study of De Martino et al.Citation30 (data not shown).
We also tested the association between dual null genotype of GSTM1–GSTT1 and RCC risk. The results showed that the dual null genotype of GSTM1–GSTT1 could predict the risk of RCC in Asians, but this association was not observed in overall populations and Caucasians. The dual null genotype of GSTM1–GSTT1 might be a risk factor for RCC in Asians. However, more investigations are still required to further evaluate the interaction of GSTT1 gene polymorphism with RCC risk.
Our results indicated that GSTM1/GSTT1 null genotype was associated with the RCC susceptibility in Caucasians and Asians, and the dual null genotype of GSTM1–GSTT1 was associated with RCC risk in Asians. However, more genetic epidemiological investigations are required to further explore this relationship. However, those findings should be regarded cautiously because many other ingredients, such as language bias, small sample size of the included report, limited statistical power, heterogeneity of enrolled cases, variable study designs, and different interventions, were closely related to affect the results.
In this meta-analysis, publication bias test was performed when the number of included studies was not less than six. In our publication bias test, we found that there was no publication bias for the association of GSTM1 or GSTT1 null genotype with RCC risk in overall populations. The results for the association of GSTM1 or GSTT1 null genotype with RCC risk in overall populations might be robust to some extent. However, the number of included studies was also a little small (only six), and more studies should be performed in the future.
In conclusion, the results from our study support that the null genotype of GSTM1/GSTT1 is associated with the risk of RCC in Caucasians and Asians, and there is an association between the dual null genotype of GSTM1–GSTT1 and the risk of RCC in Asians. However, more association investigations are required to further clarify the role of the null genotype of GSTM1/GSTT1 in predicting the risk of RCC.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Akagi EM, Junior PL, Simons SM, Bellini MH, Barreto SA, Chudzinski-Tavassi AM. Pro-apoptotic effects of Amblyomin-X in murine renal cell carcinoma “in vitro”. Biomed Pharmacother. 2012;66:64–69.
- Gao H, Dong B, Jia J, . Application of ex vivo (1)H NMR metabonomics to the characterization and possible detection of renal cell carcinoma metastases. J Cancer Res Clin Oncol. 2012;138:753–761.
- Chu H, Wang M, Yan F, . Polymorphisms in the IL-13 and IL-4R genes are associated with the development of renal cell carcinoma. Ann Oncol. 2012. doi:10.1093/annonc/mdr607
- Ahmad ST, Arjumand W, Seth A, . Risk of renal cell carcinoma and polymorphism in phase I xenobiotic metabolizing CYP1A1 and CYP2D6 enzymes. Urol Oncol. 2012. doi:10.1016/j.urolonc.2011.12.009
- Arjumand W, Ahmad ST, Seth A, Saini AK, Vitamin SS. D receptor FokI and BsmI gene polymorphism and its association with grade and stage of renal cell carcinoma in North Indian population. Tumour Biol. 2012;33:23–31.
- Zhang L, Qiu L, Wu H, . Expression profiles of seven glutathione S-transferase (GST) genes from Venerupis philippinarum exposed to heavy metals and benzo[a]pyrene. Comp Biochem Physiol C Toxicol Pharmacol. 2012;155:517–527.
- Datta SK, Kumar V, Pathak R, . Association of glutathione S-transferase M1 and T1 gene polymorphism with oxidative stress in diabetic and nondiabetic chronic kidney disease. Ren Fail. 2010;32:1189–1195.
- Salah GB, Kallabi F, Maatoug S, . Polymorphisms of glutathione S-transferases M1, T1, P1 and A1 genes in the Tunisian population: An intra and interethnic comparative approach. Gene. 2012;498:317–322.
- Langevin SM, Ioannidis JP, Vineis P, Taioli E. Assessment of cumulative evidence for the association between glutathione S-transferase polymorphisms and lung cancer: Application of the Venice interim guidelines. Pharmacogenet Genomics. 2010;20:586–597.
- Safarinejad MR, Shafiei N, Safarinejad SH. Glutathione S-transferase gene polymorphisms (GSTM1, GSTT1, GSTP1) and prostate cancer: A case-control study in Tehran, Iran. Prostate Cancer Prostatic Dis. 2011;14:105–113.
- Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Association of genetic polymorphism of glutathione S-transferase (GSTM1, GSTT1, GSTP1) with bladder cancer susceptibility. Urol Oncol. 2011. doi:10.1016/j.urolonc.2011.11.027
- Ramalhinho AC, Fonseca-Moutinho JA, Breitenfeld L. Glutathione S-transferase M1, T1, and P1 genotypes and breast cancer risk: A study in a Portuguese population. Mol Cell Biochem. 2011;355:265–271.
- Chen YL, Tseng HS, Kuo WH, Yang SF, Chen DR, Tsai HT. Glutathione S-transferase P1 (GSTP1) gene polymorphism increases age-related susceptibility to hepatocellular carcinoma. BMC Med Genet. 2010;11:46.
- Qin YH, Zhou TB, Su LN, Lei FY, Zhao YJ, Huang WF. The efficacy of different dose intravenous immunoglobulin in treating acute idiopathic thrombocytopenic purpura: A meta-analysis of 13 randomized controlled trials. Blood Coagul Fibrinolysis. 2010;21:713–721.
- Zhou TB, Qin YH, Su LN, . Insertion/deletion (I/D) polymorphism of angiotensin-converting enzyme gene in steroid-resistant nephrotic syndrome for children: A genetic association study and meta-analysis. Ren Fail. 2011;33:741–748.
- Zhou TB, Liu YG, Lin N, . Relationship between angiotensin-converting enzyme insertion/deletion gene polymorphism and systemic lupus erythematosus/lupus nephritis: A systematic review and metaanalysis. J Rheumatol. 2012; 39:686–693.
- Zhou TB, Qin YH, Su LN, . The association between angiotensin-converting enzyme insertion/deletion gene variant and risk of focal segmental glomerulosclerosis: A systematic review and meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011;12:624–633.
- Zhou TB, Qin YH, Su LN, Lei FY, Huang WF, Zhao YJACE. I/D gene polymorphism can’t predict the steroid responsiveness in Asian children with idiopathic nephrotic syndrome: A meta-analysis. PLoS One. 2011;6:e19599.
- Zhou TB, Qin YH, Ou C, . A meta-analysis of the association between angiotensin-converting enzyme insertion/deletion gene polymorphism and steroid-sensitive nephrotic syndrome in children. J Renin Angiotensin Aldosterone Syst. 2012;13:175–183.
- Zhou TB, Ou C, Qin YH, . Association of angiotensin converting enzyme insertion/deletion gene polymorphism with idiopathic nephrotic syndrome susceptibility in children: A meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011;12:601–610.
- Zhou TB, Lin N, Liu YG, Qin YH, Shao MB, Peng DD. Association of ACE I/D gene polymorphism with vesicoureteral reflux susceptibility in children: A meta-analysis. J Renin Angiotensin Aldosterone Syst. 2012;13:273–281.
- Wang X, Jie YW. Timing of initiation of renal replacement therapy in acute kidney injury: A systematic review and meta-analysis. Ren Fail. 2012;34(3):396–402.
- He Q, Zhang W, Chen J. A meta-analysis of icodextrin versus glucose containing peritoneal dialysis in metabolic management of peritoneal dialysis patients. Ren Fail. 2011;33:943–948.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101.
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634.
- Sweeney C, Farrow DC, Schwartz SM, Eaton DL, Checkoway H, Vaughan TL. Glutathione S-transferase M1, T1, and P1 polymorphisms as risk factors for renal cell carcinoma: A case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:449–454.
- Bruning T, Lammert M, Kempkes M, Thier R, Golka K, Bolt HM. Influence of polymorphisms of GSTM1 and GSTT1 for risk of renal cell cancer in workers with long-term high occupational exposure to trichloroethene. Arch Toxicol. 1997;71: 596–599.
- Longuemaux S, Delomenie C, Gallou C, . Candidate genetic modifiers of individual susceptibility to renal cell carcinoma: A study of polymorphic human xenobiotic-metabolizing enzymes. Cancer Res. 1999;59:2903–2908.
- Karami S, Boffetta P, Rothman N, . Renal cell carcinoma, occupational pesticide exposure and modification by glutathione S-transferase polymorphisms. Carcinogenesis. 2008;29: 1567–1571.
- De Martino M, Klatte T, Schatzl G, . Renal cell carcinoma Fuhrman grade and histological subtype correlate with complete polymorphic deletion of glutathione S-transferase M1 gene. J Urol. 2010;183:878–883.
- Ahmad ST, Arjumand W, Seth A, Saini AK, Sultana S. Impact of glutathione transferase M1, T1, and P1 gene polymorphisms in the genetic susceptibility of North Indian population to renal cell carcinoma. DNA Cell Biol. 2012;31:636–643.
- Sun QP, Li LY, Chen Z, . Detection of TMPRSS2-ETS fusions by a multiprobe fluorescence in situ hybridization assay for the early diagnosis of prostate cancer: A pilot study. J Mol Diagn. 2010;12:718–724.
- Jung S, Jeong D, Kim J, . The role of hLHX6-HMR as a methylation biomarker for early diagnosis of cervical cancer. Oncol Rep. 2010;23:1675–1682.
- Moribe T, Iizuka N, Miura T, . Identification of novel aberrant methylation of BASP1 and SRD5A2 for early diagnosis of hepatocellular carcinoma by genome-wide search. Int J Oncol. 2008;33:949–958.
- Gong M, Dong W, An R. Glutathione S-transferase T1 polymorphism contributes to bladder cancer risk: A meta-analysis involving 50 studies. DNA Cell Biol. 2012. doi:10.1089/dna.2011.1567
- Zhang ZJ, Hao K, Shi R, . Glutathione S-transferase M1 (GSTM1) and glutathione S-transferase T1 (GSTT1) null polymorphisms, smoking, and their interaction in oral cancer: A HuGE review and meta-analysis. Am J Epidemiol. 2011;173:847–857.
- Chen B, Cao L, Zhou Y, . Glutathione S-transferase T1 (GSTT1) gene polymorphism and gastric cancer susceptibility: A meta-analysis of epidemiologic studies. Dig Dis Sci. 2010;55:1831–1838.
- Gao LB, Pan XM, Li LJ, . Null genotypes of GSTM1 and GSTT1 contribute to risk of cervical neoplasia: An evidence-based meta-analysis. PLoS One. 2011;6:e20157.
- Xu D, Yan S, Yin J, Zhang P. Null genotype of GSTT1 contributes to colorectal cancer risk in Asian populations: Evidence from a meta-analysis. Asian Pac J Cancer Prev. 2011;12: 2279–2284.
- Zhong S, Yang JH, Liu K, Jiao BH, Chang Z. Null genotype of glutathione S-transferase Tl contributes to colorectal cancer risk in the Asian population: A meta-analysis. J Gastroenterol Hepatol. 2012;27:231–237.
- Liao C, Cao Y, Wu L, Huang J, Gao F. An updating meta-analysis of the glutathione S-transferase T1 polymorphisms and colorectal cancer risk: A HuGE review. Int J Colorectal Dis. 2010;25:25–37.