Abstract
A case of granulomatous interstitial nephritis (GIN) associated with Crohn’s disease (CD) was reported. GIN is a rare pathological finding in renal biopsy specimens. In a patient affected by CD, granulomas may be found in various tissues and organs such as lymph nodes, mesentery, liver, and lungs and occasionally in bones, joints, and skeletal muscle. Few cases of granuloma have been reported in the kidney, and it is not always possible to relate the presence of granuloma to CD, to other interstitial granulomatosis diseases, or to a drug-induced reaction. The issue has a remarkable clinical effect; indeed, the answer requires a completely different therapeutic approach. The diagnosis analysis on the basis of clinical–pathological evidences and on reports from literature is discussed.
INTRODUCTION
Renal involvement in patients affected by Crohn’s disease (CD) is quite common and has different origins. These patients are quite often affected by calcium oxalate and uric acid stones due to steatorrea and diarrhea or by a glomerular disease. Moreover, a silent clinical chronic renal impairment may occur and a picture of chronic interstitial nephritis is present and is generally associated with the use of anti-inflammatory medications like mesalazine. Rare cases are reported in which it is possible to find a granuloma formation in the kidney as well as in the intestinal tissue.Citation1
We report an unusual case characterized clinically by a quite rapid deterioration of renal function and histologically by the presence of numerous interstitial granuloma.
CASE REPORT
In 2002, CD was diagnosed in a 49-year-old man and a successful treatment with mesalazine was started.
Three years later, there was a relapse and the patient was treated with prednisone, azatioprine, and mesalazine (1600 mg) twice a day. In 2008, renal function was normal: serum creatinine was 1.1 mg/dL and urinalysis was negative.
One year later, he was admitted to our ward due to a rapid deterioration of renal function. Serum creatinine had risen from 1.1 mg/dL to 2.9 mg/dL in 4 months. A physical examination proved normal, blood pressure was 120/75 mmHg, and laboratory examinations showed BUN 79 mg/dL, serum creatinine 3.1 mg/dL, estimated Glomerular Filtration Rate (eGFR) (MDRD) 24 mL/min/1.73 m2, uric acid 6.2 mg/dL, and calcemia 9.3 mg/dL. A non-nephrotic proteinuria was detected (620 mg/g urinary creatinine) with increased excretion of alpha-1-microglobulin (92 mg/L; normal range < 0.12 mg/L). Urinary sediment also showed 10–15 red blood cells with high power field (HPF). The 24-h diuresis was 1.900 mL.
Moreover, normocytic and normochromic anemia was present: RBC 3.32 × 106/mm3, Hb 10.7 g/dL, Ht 27.7%, WBC count 5.4 × 109/mm3, plateletes (PTLs) 311 × 103/mm3, Erythrocyte Sedimentation Rate (ESR) 41 mm/h, and serum albumin 4.04 g/dL.
Tests for Brucella, Mycobacterium tuberculosis, and auto-antibodies (ANA, ENA, and ANCA) proved negative. Serum level of angiotensin-converting enzyme resulted in the normal range: 26 UI (normal range 18– 55 UI).
The chest X-ray and a renal ultrasound were normal. An interstitial nephritis due to mesalazine was suspected and the patient underwent a renal biopsy.
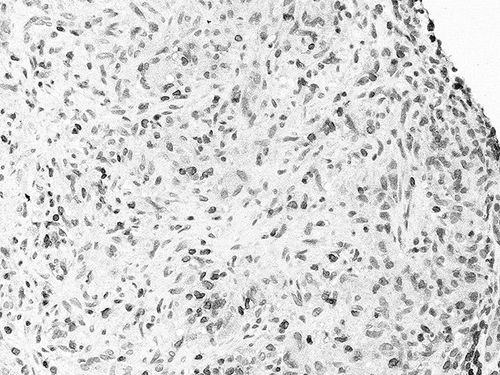
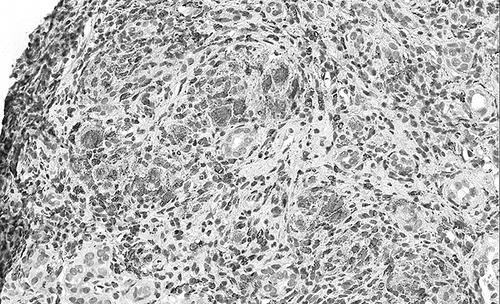
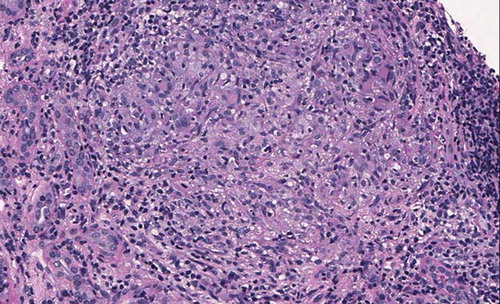
The renal biopsy contained 32 glomeruli without histological lesions and the direct immune fluorescence was negative for immunoglobulins and complement fractions. Arterial walls showed a diffused fibrosis with elastic lamina reduplication. A severe, diffused, inflammatory cell interstitial infiltrate was detected and, in addition, the infiltrating cells were arranged as granuloma: there was an outer circular layer composed of multinucleated giant cells (). The infiltrate was made up of variable numbers of lymphocytes (mostly CD3+ T cells) (), monocytes/macrophages (CD68+) (), and plasma cells. There was no caseous necrosis, and the Ziehl–Neelsen staining was negative.
Figure 1. Light microscopy: a granuloma composed of lymphocytes, monocytes, and some multinucleated giant cells is well evident in the interstitial tissue (PAS 400×).
There were only rare eosinophil leucocytes and no crystal or polarized material was present. In addition, there was a focal tubular atrophy associated with interstitial fibrosis. In some areas, the CD3+ cells were infiltrating the tubular walls (tubulitis).
Following discharge the patient was treated with high doses of steroids and subsequently with anti-tumor necrosis factor antibodies, but renal function continued to worsen and renal replacement therapy was started 1 year after the renal biopsy.
DISCUSSION
Granulomatous interstitial nephritis (GIN) is a rare condition whose pathogenesis is poorly understood.Citation2 A rare case of GIN was reported in a patient affected by a severe form of CD. The renal involvement was not affected at all by any treatment and led to severe renal failure requiring renal replacement treatment with hemodialysis.
GIN is rarely found in patients who have undergone a renal biopsy. The literature figures report an incidence variable from 0.9% to 6% according to different authors.Citation3 There can be a number of causes () and drugs are most frequently involved (37.5%). The most common drugs are antibiotics, diuretics, and others such as anti-inflammatory nonsteroids or antihypertensive drugs. Infections (M. tuberculosis, histoplasma), foreign body reaction, crystal of monosodium urate or oxalate precipitation stimulate a giant cell reaction. Furthermore, even with immune processes such as sarcoidosis, tubulointerstitial nephritis, and uveitis syndrome or in necrotizing vasculitis such as Wegener disease, it is possible to find interstitial granuloma. Actually, in Wegener disease, most of the granuloma are made of cellular infiltrate with epithelioid cells surrounding the glomeruli with necrotizing extracapillary glomerulonephritis and disruption of the Bowman’s capsule (granulomatous-like lesion) and interstitial necrotizing granulomatous inflammation are not very frequent.Citation4,5 Other interstitial granuloma is associated with cholesterol emboli derived from atheromatous lesions of the abdominal aorta and in which it is easy to recognize the needle-shaped crystals of cholesterol. In some cases, it is not possible to recognize a cause: the so-called idiopathic granulomatous nephritis.Citation6
Table 1. Granulomatous interstitial nephritis.
Moreover, the susceptibility to be affected by CD has been correlated with Nod2 gene mutation and also in relationship with TL1 (TNFSF15)/DR3 axis. However, we were not able to perform a genetic investigation on our patient. To our knowledge, no correlation with these alterations and the extra-intestinal localization of Crohn has been described so far.Citation7
In literature, sporadic cases of GIN in CD have been described.Citation8–11 In all of these reports, the problem is whether the renal interstitial granulomas are due to the extra-intestinal involvement of CD or to the treatments for CD. Indeed, in 1% of patients being treated with 5-ASA (mesalazine), among one of the most used drugs in CD, renal impairment occurs. In a few cases, a renal biopsy documented a severe interstitial nephritis, but granulomas were never described.Citation12,13 The incidence of interstitial nephritis is at its maximum during the first year of treatment and the withdrawal of 5-ASA is followed by an increase in renal function. In the cases where there is no improvement of renal function following the withdrawal of the drug, a cycle of treatment with high doses of steroids can be useful.
The first report of GIN in a patient with CD was by ArchimandritisCitation8 who described a patient who had never been given 5-ASA and in whom a remarkable clinical improvement came about following a colonostomy. The second reportCitation8 deals with a patient in whom GIN developed 19 months after the withdrawal of 5-ASA, while in the third,Citation9 GIN occurred at the same time as the treatment with 5-ASA. Finally, in the case report by Unal,Citation11 GIN was diagnosed 12 months after the discontinuation of 5-ASA. In all cases, the withdrawal of 5-ASA was followed by a treatment with steroids; some clinical benefits were seen only in the last case.
In these cases,Citation8–11 GIN as an extra-intestinal manifestation of CD has been proposed. It has been speculated that colon and extra-colon organs have common antigens and that the immunoreactions against diffused cellular antigen play a central role in extra-renal manifestations in CD.Citation6
In the case described, we can presume a causative role of 5-ASA (renal impairment occurred during treatment), but at the same time there was no recovery of renal function following the withdrawal of neither mesalazine nor the steroid therapy. Moreover, to our knowledge, granulomas due to 5-ASA therapy were never described. Other causes of GIN such as sarcoidosis or TBC were excluded.
Taking everything into consideration, we have made a final diagnosis of GIN attributing renal granuloma as an extra-renal manifestation of CD.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006;12:4819–4831.
- Viero RM, Cavallo T. Granulomatous interstitial nephritis. Hum Pathol. 1995;26:1347–1353.
- Mignon F, Mèry JPH, Mongenot B. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp. 1984;13:219–245.
- Jennette JC, Thomas DB. Pauci-immune and antineutrophil cytoplasmatic antibody mediated crescentic glomerulonephritis and vasculitis. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, eds. Hepinstall Pathology of the Kidney. 6th ed. New York: Lippincott Williams and Wilkins; 2007:644–672.
- Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol. 2007;2:222–230.
- Waters AM, Zachos M, Herzenberg AM, Harvey E, Rosenblum ND. Tubulointerstitial nephritis as an extraintestinal manifestation of Crohn’s disease. Nat Clin Pract Nephrol. 2008;4:693–697.
- Siggers RH, Hackam DJ. The role of innate immune-stimulated epithelial apoptosis during gastrointestinal inflammatory diseases. Cell Mol Life Sci. 2011;68(22):3623–3634.
- Archimandritis AJ, Weetch MS. Kidney granuloma in Crohn’s disease. BMJ. 1993;28(307):540–541.
- Baumer P, Brocard JF, Kohn W, Bedossa P, Charpentier B. Severe chronic interstitial nephritis associated with Crohn’s disease, but not with mesalazine? Gastroenterol Clin Biol. 1999;23:1400–1402.
- Mahmood A, Poller DN, Ramage JK. Are renal microgranulomas related to inflammatory tubular estruction? J Clin Pathol. 2004;57:551–552.
- Unal A, Sipahioglu MH, Akgun H, . Crohn’s disease complicated by granulomatous interstitial nephritis, choroidal neovascularization, and central retinal vein occlusion. Intern Med. 2008;47:103–107.
- Schwarz A, Krause PH, Keller F, Offermann G. Mihatsch: Granulomatous interstitial nephritis after nonsteroidal anti-inflammatory drugs. Am J Nephrol. 1988;8:410–416.
- Gisbert JP, Gonzales-Lama Y, Matè J. 5-Aminosalicylates and renal function in inflammatory bowel disease: A systematic review. Inflamm Bowel Dis. 2007;5:629–638.