Abstract
We report on the isolated unilateral renal venous thrombosis (RVT) detected in a young patient who used vibration belt to stay thin. Apart from her sickle cell trait, the patient presented no other clinical situations associated with RVT.
CASE REPORT
A 23-year-old female patient presented severe pain in the left flank while taking a deep breath. Occurrences of fever, nausea, vomiting, gross hematuria, and edema were denied. Physical examination showed that blood pressure was 100/60 mmHg, pulse was 96/min, body temperature was 36.9°C, body weight was 54 kg, height 177 cm, body mass index (BMI) was 17.24 kg/m2, and lung–heart sounds were normal. Abdominal rebound defense and hepatosplenomegaly were not found; there was no peripheral edema and peripheral pulses were normal. She had no history of trauma and was not on any medications. She got to know about her sickle cell trait during a screening a year earlier. Laboratory measurements produced the following results: white blood cell 8730 U/L, hemoglobin 10.8–11.7 g/dL, hematocrit 32–36%, mean corpuscular volume (MCV) 87.8/fl, platelets 212,000–460,000 u/L, red cell distribution width (RDW) 15.6%, erythrocyte sedimentation rate 67 mm/h, blood urine nitrogen (BUN) 21–23 mg/dL, creatinine 0.7–0.66 mg/dL, albumin 3.8 g/dL, total bilirubin 0.9 mg/dL, aspartate aminotransferase (AST) 45 U/L (0–31), alanine aminotransferase (ALT) 56 U/L (0–41), alkaline phosphatase 166 U/L 5–240, gamma glutamyl transpeptidase (GGT) 28 U/L (7–32), amylase 48 U/L, Na 140 mmol/L (135–145), K 3.6 mmol/L (3.5–5.1), Ca 9.3 mg/dL (8.8–10.2), total cholesterol 183 mg/dL (200), high density lipoprotein (HDL)-cholesterol 53.5 mg/dL (>40), and low density lipoprotein (LDL)-cholesterol 118 mg/dL (160). Urine analysis produced the following results: protein negative (−), glucose (−), ketone (−), microscopy 2 erythrocyte/high power field (HPF), and leukocyte 9/HPF. Other laboratory tests produced the following results: serum ferritin 229 ng/mL (11–306), free T3 2.35 pg/mL (2.3–4.2), free T4 1.14 ng/dL (0.61–1.12), thyroid stimulating hormone (TSH) 2.85 mIU/L (0.34–5.6), C reactive protein (CRP) 24.4 mg/dL (0–0.8), folate 3.77 ng/mL, vitamin B12 264.08 pg/mL (126–505), Brucella agglutinin (−), Salmonella agglutinin (−), Anticardiolipin antibody (ACA) IgM 4.21 IU/mL (0–7 IU/mL), ACA IgG 2.6 IU/mL (0–1 IU/mL), factor V Leiden homozygous, hepatitis B surface antigen (HBsAg) (–), anti hepatitis C virus (anti-HCV) (–), antinuclear antibody (–), anti-double-stranded DNA (–), antithrombin 3 107% (69–122), protein C 63% (78–134), and free protein S (fPS) 157–77 (55–160). The upper and lower gastrointestinal endoscopic examinations and the echocardiographic examination produced normal results. Lung X-ray and electrocardiography were found to be in normal limits. Then, contrast-enhanced triphasic computerized tomography (CT) was performed in dynamic mode. In this examination, the main renal arteries and the main renal veins were found to be normal bilaterally. There was a small (16 mm diameter) focal heterogeneous hypodense zone in the anterolateral cortex of the left kidney in the arterial phase (A), which became evident in the venous (nephrogram) phase (B). In pyelogram phase images, this hypodense zone became evident: when the margins of this perfusion defect were sharpened by expanding them to 34 mm, the hypodense zone became even more evident (C). The decreased nephrographic attenuation and loss of corticomedullary differentiation detected in this CT examination were compatible with focal cortical abscess and/or partial thrombus in renal cortical vein (either in lobular vein or in arcuate vein). For RVT she was treated with Nadroparin (5700 IU/day) for 1 month and after that Coumadin 5 mg/day (the target international normalized rate was 2). Following the anticoagulant treatment (50 days after the first CT examination), a control triphasic CT examination was performed. In this examination, the focal cortical perfusion defect disappeared completely and both the kidneys were reported as completely normal (A–C).
Figure 1. Focal heterogeneous hypodense zone in the anterolateral cortex of the left kidney in arterial phase (A), which became evident in the venous (nephrogram) phase (B). In pyelogram phase images, this hypodense zone became evident: when the margins of this perfusion defect were sharpened by expanding them to 34 mm, the hypodense zone became even more evident (C) (arrows point at the renal vascular damage lesion).
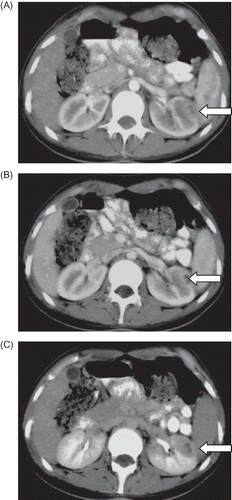
Figure 2. (A)–(C) Following the anticoagulant treatment, a control triphasic CT examination was performed. In this examination, the focal cortical perfusion defect disappeared completely and both the kidneys were reported as completely normal.
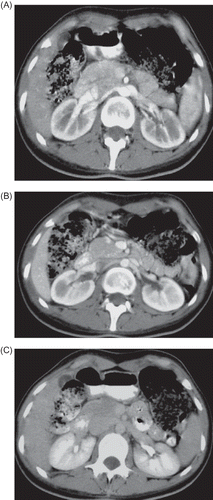
Thrombophilia or other clinical problems associated with RVT were not found in our patient. However, she used the Acura AC743 motor vibration belt on her abdomen for 1 hour a day and to every lumbar region for 45 minutes a day in order to stay thin. With 50 days of anticoagulant therapy, regression of RVT showed up in control CT examination.
DISCUSSION
RVT may be defined as the thrombus of major renal veins and their tributaries. RVT can be asymptomatic or may cause complications. RVT is often bilateral and develops as a condition secondary to an underlying clinical condition, and it develops more frequently in men. In the cases of unilateral thrombosis, the left renal vein is affected more commonly than the right, as in our case. RVT sometimes gets cured through spontaneous recovery without causing any complications, such as hypertension or kidney failure.Citation1
Since there are no specific clinical findings and suitable diagnostic laboratory tests available for the diagnosis and treatment of RVT, the best diagnostic method available so far is imaging. Radiological findings for RVT vary according to the speed of onset and degree of acclusion of the renal vein. The affected kidney enlarges in cases of a rapid onset of RVT, and full occlusion occurs and the kidney reaches a maximum diameter in a week. But following that, there will be a gradual reduction in size of the kidney in the next few weeks. Hyperechogenic enlarged kidneys in the acute phase are determined by renal ultrasonography in 90% of the patients. Color Doppler is also a useful diagnostic technique for RVT. In intravenous urography, the collecting system cannot be displayed in cases with sudden and complete occlusion.Citation2 CT scan is the most preferred imaging method. The available diagnostic methods of RVT are speedy and accurate. With intravenous contrast given at the same time, it is possible to display the renal veins.Citation3 In our hospital we detected RVT by CT angiography in our patients. RVT with magnetic resonance (MR) angiography can be diagnosed quickly and without nephrotoxicity and radiation.Citation4 A definitive diagnosis is reached using inferior vena cavagraphy and selective renal venography. These are invasive methods and may cause endothelial damage, so these methods are not preferred.Citation5 Diethylenetriamine pentaacetic acid (DTPA) renal scintigraphy and renal biopsy may also be informative.
In addition to diagnosis of RVT, the underlying clinical situation must be investigated and determined. The underlying clinical situation may be endothelial damage such as blunt trauma, venography, tumor infiltration, vasculitis, homocystinuria, renal transplantation, and damaged endothelium; stasis such as excessive fluid loss, interventions of the retroperitoneal region, distortion of the renal vein, and kink; or thrombophilia such as nephrotic syndrome, sepsis, puerperium, malignant diseases, oral contraceptives and factor V Leiden the prothrombin gene mutation (G 20210A), protein S—protein C—antithrombin deficiencies, antiphospholipid syndrome, systemic lupus erythematosus, and Behçet’s disease. It is in her medical history that a 12-week pregnancy was terminated with abortion for unknown reasons 1 year ago, and then the sickle cell trait was found. To avoid becoming fat and losing the thin appearance, she was using vibration belt. In fact by the application of ultrasonography, arterial and venous thromboses usually dissolve. It has been found in experimental studiesCitation6 that an ultrasonic angioplasty device can be used effectively for thrombus in patients with acute myocardial infarcts and, without acute atrial fibrillation, transthoracic sonic vibration cleared acute coronary thrombus.Citation7 In light of this information, the relationship between RVT and the vibration caused by a vibration belt can be explained in terms of the compression effect of the belt and/or low BMI of the patient. In our case, a splenic vein thrombosis following abdominal compression and vibration and an isolated renal vein thrombosis after blunt trauma were reported.Citation8,9
An anticoagulant therapy resulted in a complete regression of RVT in 50 days in our patient without any complication. Sickle cell disease can cause renal damage, but sickle cell trait has not been reported so far to cause RVT or other renal damages.
The following is the summary of our results:
| (1) | An unilateral RVT developed without renal parenchymal damage or arterial thrombosis in our patient. | ||||
| (2) | The cause of RVT could not be determined. Her medical history shows the use of vibration belt. Although ultrasonic energy can actually lead to the dissolution of a thrombus, the occurrence of belt vibration may be associated with RVT, especially in patients with low BMI. We did not find a similar report in the relevant literature in English. | ||||
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Asghar M, Ahmed K, Shah SS, Siddique MK, Dasgupta P, Khan MS. Renal vein thrombosis. Eur J Vasc Endovasc Surg. 2007;34(2):217–223.
- Ricci MA, Lloyd DA. Renal venous thrombosis in infants and children. Arch Surg. 1990;125(9):1195–1199.
- Alvarez-Castels A, Sebastia CC, Quiroga GS. Computerized tomography angiography of renal vessels. Arch Esp Urol. 2001; 54(6):603–615.
- Hodgson D, Rankin S, Jan W, Koffman G, Khan MS. Magnetic resonance imaging of living related kidney donor – An analysis of 111 consecutive cases. BJU Int. 2006;97(3):584–586.
- Kuntz RM, Schutz W, Becker HM. Traumatic renal vein thrombosis. Med Klin. 1981;76(21):595–596.
- Drobinski G, Philippe F, Bucherer C, . Effects of ultrasonic energy on blood clots in vitro. Arch Mal Coeur Vaiss. 1993; 86(6): 915–920.
- Hoffmann AK, Gill H. A study to determine chest wall vibratory attachment interface locations for a low frequency sonic vibrator in treatment of acute coronary thrombosis. J Thromb Thrombolysis. 2011;32(2):167–176.
- Tzur I, Almoznino-Sarafian D, Dotan E, . Splenic vein thrombosis following abdominal compression and vibration: A case report. Angiology. 2008;59(4):514–516.
- Kau E, Patel R, Fiske J, Shah O. Isolated renal vein thrombosis after blunt trauma. Urology. 2004;64(4):807–808.
