Abstract
Background: Acquired immunity is impaired in hemodialysis (HD) patients, and decreased T-cell number may contribute. Indoleamine 2,3-dioxygenase (IDO) and arginase type I (ARG) catabolize tryptophane and arginine, respectively, and exert proapoptotic and antiproliferative effects on T-cells. Plasma levels of IDO and ARG and their relation to blood T-cell number were evaluated in HD patients. Methods: Thirty-two HD patients and 20 healthy controls participated in the study. Plasma IDO and ARG were measured by means of enzyme-linked immunosorbent assay. T-cell number was assessed by means of flow cytometry. Results: IDO concentration was significantly higher in HD patients than in healthy volunteers (44.30 ± 31.83 ng/mL vs. 21.28 ± 26.21 ng/mL, p = 0.009). There was a trend for higher ARG concentration in HD patients (13.43 ± 11.91 ng/mL) than in healthy volunteers (9.56 ± 4.03 ng/mL), which, however, did not reach statistic significance (p = 0.099). Absolute T-cell count was significantly lower in HD patients than in healthy controls (1176.99 ± 567.71 cells/mm3 vs. 1519.85 ± 594.96 cells/mm3, p = 0.040). Absolute blood T-cell number was inversely related to plasma IDO (r = −0.490, p = 0.004) and to plasma ARG (r = −0.387, p = 0.029) concentrations. Conclusions: Plasma IDO and ARG may contribute to decreased blood T-cell count in HD patients.
INTRODUCTION
Acquired immunity is impaired in hemodialysis (HD) patients, a phenomenon that is associated with the inflammatory state of these patients.Citation1 High frequency of negative, delayed-type hypersensitivity skin testsCitation2,3 and high failure rates of vaccination with T-cell-dependent peptide antigens,Citation4 but not with T-cell-independent polysaccharide antigens,Citation5 clearly indicate that T-cell function is impaired in HD patients.
Indoleamine 2,3-dioxygenase (IDO) catalyzes the initial rate-limiting step of tryptophan degradation along the kynurenine pathway. IDO is inducible by various inflammatory stimuli. Its expression in antigen presenting cells (APCs) is accompanied by impaired adaptive immune response because tryptophan depletion and kynurenine pathway products in local microenvironment decrease T-cell proliferation, increase T-cell apoptosis, and induce the emergence of regulatory T-cells (Tregs). IDO-mediated immunosuppression reduces transplantation rejection and ameliorates the clinical course of various experimental autoimmune diseases. Inhibition of T-cell function via IDO is also mediated by non-APC cell types. Expression of IDO in paternally derived placental trophoblast contributes to success of semi-allogenic pregnancy, whereas IDO expression by tumor cells contributes to escape of tumors by immunosurveillance.Citation6–11 Because IDO induces T-cell apoptosis and reduces T-cell proliferation, it is not surprising that its increased plasma activity has been found to be inversely related to blood T-cell count in patients suffered from sepsis.Citation12 In these patients, response to treatment of severe sepsis was accompanied by significant decrease of IDO activity.
Arginase type I (ARG) catabolizes arginine to ornithine and urea and its expression in myeloid-derived suppressor cells (MDSCs) has been incriminated for impaired immune response in situations such as cancer, certain infections, and trauma. In the above conditions, MDSCs are accumulated not only in the local microenvironment but also in peripheral lymphoid organs inducing systemic immunosuppression.Citation10,13 Depletion of arginine in proximity to T-cells induces impaired signaling using the antigen-specific T-cell receptor because of selective zeta-chain downregulationCitation14 and also affects the capacity of T-cells to proliferate by decreasing the expression of the cell cycle regulators cyclin D3 and cyclin-dependent kinase 4.Citation15 Interestingly, T-cell receptor complex zeta-chain downregulation has been confirmed in HD patients.Citation16 Recent experimental data support that MDSCs promote T-cell apoptosis as well.Citation17,18 Because ARG affects T-cell proliferation and induces T-cell apoptosis, it is not surprising that its peripheral activity has been found to be inversely related to blood T-cell count in patients suffered from cancer.Citation19 In these patients, ARG activity was decreased significantly after surgical resection of tumor, along with the circulating CD16+ cells, which were playing the role of MDSCs.
In HD patients, T-cell count is decreased and increased apoptosis as well as decreased proliferation have been confirmed.Citation20–22 Considering the proapoptotic and the antiproliferative effects of IDO and ARG on T-cells, the plasma levels of these enzymes and their relations to blood T-cell count in HD patients were evaluated.
PATIENTS AND METHODS
Patients
Thirty-two HD patients (mean age 61.13 ± 12.74 years, 18 males) and 20 healthy volunteers (mean age 58.90 ± 6.25 years, 13 males) participated in the study. All patients were anuric and the cause of end-stage renal disease (ESRD) was primary glomerulonephritis in 9 patients, diabetes mellitus in 7 patients, hypertension in 3 patients, autosomal dominant polycystic kidney disease in 2 patients, obstructive nephropathy in 2 patients, analgesic nephropathy in 1 patient, cholesterol embolism in 1 patient, Alport’s syndrome in 1 patient, and unknown in 6 patients.
Patients underwent regular HD with polysulfone low-flux dialyzers and bicarbonate buffer for 4 h sessions, 3 times a week, and for at least 1 year prior to the study. The mean urea reduction ratio was 64.98 ± 7.22%. Mean hemoglobin was 11.24 ± 1.07 g/dL. Mean serum intact parathyroid hormone was 250.05 ± 211.90 pg/mL, calcium 9.60 ± 0.65 mg/dL, and phosphorous 5.87 ± 1.74 mg/dL. Serum albumin was 3.96 ± 0.34 g/dL, body mass index 24.40 ± 5.34 kg/m2, and normalized protein catabolic rate 1.22 ± 0.17 g/kg/day. C-reactive protein (CRP) was 9.66 ± 14.71 mg/L. None of the patients were hepatitis B, hepatitis C, or human immunodeficiency virus carrier or suffered from acute infection, malignancy, or autoimmune disease. Because ARG is abundantly expressed in the liver,Citation23 the patients were selected to be free of any liver disease. Due to the hypotransaminasemia that characterizes HD patients, those with serum alanine aminotransferase (ALT) higher than 24 IU/L and/or aspartate aminotransferase (AST) higher than 17 IU/L were excluded from the study.Citation24 Finally, none of the patients were receiving cytotoxic drugs, corticosteroids, or nonsteroidal, anti-inflammatory drugs. Patients’ clinical characteristics are provided in .
Table 1. Patients’ clinical characteristics.
An informed consent was obtained from each individual enrolled in the study and the hospital ethics committee gave its approval to the study protocol.
Blood T-Cell Count
Blood samples were drawn just before the onset of the second dialysis session of the week. Absolute lymphocyte number was measured and absolute T-cell count was estimated from the T-cell percentage assessed by means of flow cytometry performed by EPICS XL-MCL apparatus (Beckman Coulter, Inc., Fullerton, CA, USA). T-cells were gated according to the surface expression of CD3-epsilon using anti-CD3-epsilon phycoerythrin-Cy5 (PE-Cy5) conjugated mouse monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Plasma IDO and Arginase Type I
Blood samples were drawn just before the onset of the second dialysis session of the week and at the same time with the blood collection for flow cytometry analysis. Harvested plasma was stored at −20°C.
Plasma IDO was measured by means of enzyme-linked immunosorbent assay (ELISA) (CUSABIO Biotech Co., Wuhan, China). The analytical limit of detection of the above ELISA kit is 0.195 ng/mL. Plasma ARG was measured using an ELISA kit (Biovendor GmbH, Heidelberg, Germany). The analytical limit of detection of the above ELISA kit is 0.5 ng/mL.
Statistical Analysis
One sample Kolmogorov–Smirnov test confirmed the normality of the evaluated variables. For comparison of means, unpaired t-test was used, whereas for evaluating relations linear regression analysis was performed. Results were expressed as mean ± SD and a p < 0.05 was considered as significant. About 95% confidence interval (CI) of difference was also calculated.
RESULTS
Plasma IDO and ARG Concentrations and Blood T-Cell Count in HD Patients and Healthy Volunteers
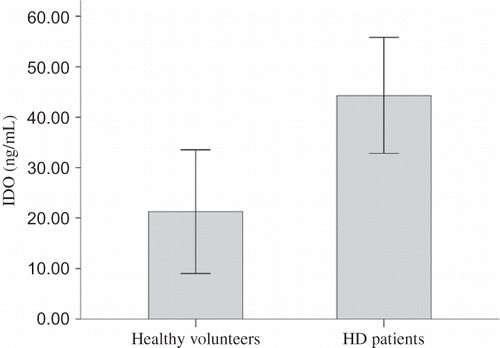
Plasma IDO concentration was significantly higher in HD patients than in healthy volunteers (44.30 ± 31.83 ng/mL vs. 21.28 ± 26.21 ng/mL, p = 0.009, 95% CI of difference from 5.96 to 40.10 ng/mL) ().
Figure 1. Plasma IDO concentration in HD patients and healthy volunteers.
Note: Plasma IDO concentration was significantly higher in HD patients than in healthy volunteers (44.30 ± 31.83 ng/mL vs. 21.28 ± 26.21 ng/mL, p = 0.009, 95% CI of difference from 5.96 to 40.10 ng/mL).
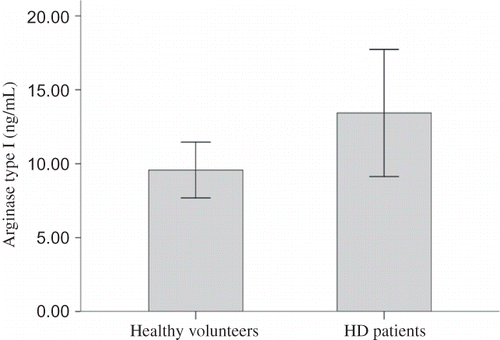
Plasma ARG concentration was 13.43 ± 11.91 ng/mL in HD patients and 9.56 ± 4.03 ng/mL in healthy volunteers. Although there was a trend for higher ARG level in HD patients, the difference did not reach statistical significance (p = 0.099, 95% CI of difference from −0.75 to 8.49 ng/mL) ().
Figure 2. Plasma ARG concentration in HD patients and healthy volunteers.
Note: There was a trend for higher plasma ARG concentration in HD patients (13.43 ± 11.91 ng/mL) than in healthy volunteers (9.56 ± 4.03 ng/mL), which however did not reach statistical significance (p = 0.099, 95% CI of difference from −0.75 to 8.49 ng/mL).
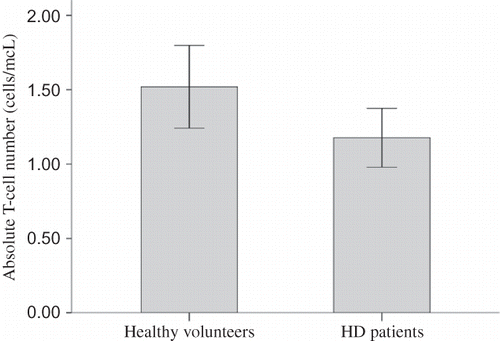
Absolute lymphocyte number did not differ significantly between HD patients and healthy volunteers (1779.41 ± 762.29 cells/mm3 vs. 2110.00 ± 704.04 cells/mm3, p = 0.120, 95% CI of difference from −749.91 to 88.73 cells/mm3). T-cell percentage was 64.95 ± 11.72% in HD patients, and significantly lower than T-cell percentage in healthy volunteers, which was 71.04 ± 7.81% (p = 0.026, 95% CI of difference from −11.44% to −0.75%). Thus, absolute T-cell count was significantly lower in HD patients than in healthy volunteers (1176.99 ± 567.71 cells/mm3 vs. 1519.85 ± 594.96 cells/mm3, p = 0.040, 95% CI of difference from −669.60 to −16.12 cells/mm3) ().
Relations between Plasma IDO and ARG Concentrations and Absolute Blood T-Cell Number in HD Patients and Healthy Volunteers
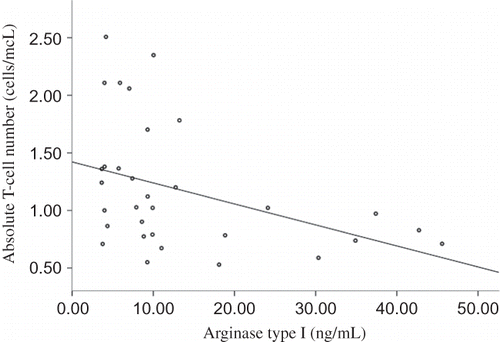
In HD patients, absolute blood T-cell number was inversely related to plasma IDO concentration (r = −0.490, p = 0.004) (). Similarly, in HD patients, absolute blood T-cell count was inversely related to plasma ARG concentration (r = −0.387, p = 0.029) ().
Figure 4. Relation between absolute blood T-cell number and plasma IDO in HD patients.
Note: Absolute blood T-cell number was inversely related to plasma IDO concentration in HD patients (r = −0.490, p = 0.004).
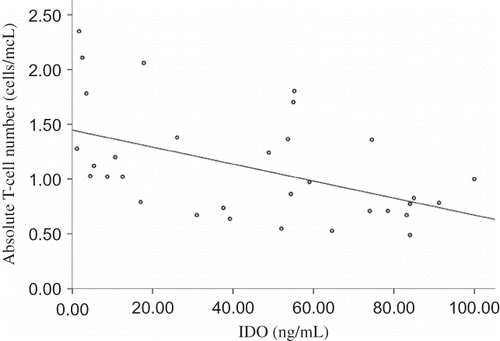
Figure 5. Relation between absolute blood T-cell number and plasma ARG in HD patients.
Note: Absolute blood T-cell count was inversely related to plasma ARG concentration in HD patients (r = −0.387, p = 0.029).
On the contrary, not such relations were detected in healthy volunteers, who had lower plasma levels of IDO and ARG. Absolute T-cell number was related neither to plasma IDO concentration (r = 0.139, p = 0.559) nor to ARG concentration (r = 0.051, p = 0.831).
DISCUSSION
Acquired immunity is impaired in HD patientsCitation1 and decreased T-cell number due to increased apoptosis and/or decreased proliferation may contribute.Citation20–22 IDO and ARG, expressed by APCs and MDSCs, respectively, in proximity to T-cells at the site of immune response and in secondary lymphoid organs, exert immunosuppressive effects by various mechanisms, apoptosis, and impaired proliferation of T-cells included.Citation6–11,13,15,17,18 In the clinic, increased circulating IDO level has been associated with decreased blood T-cell number in patients suffered from sepsis,Citation12 whereas increased circulating ARG level has been associated with decreased blood T-cell count in patients suffered from cancer.Citation19 In this study, plasma IDO and ARG levels were measured and their associations with blood T-cell number were evaluated in HD patients.
Plasma IDO concentration was significantly higher in HD patients. This is in accordance with previous studies that showed increased plasma IDO activity or concentration in this population.Citation25–28 As in a previous study,Citation29 ARG concentration does not statistically significantly differ between HD patients and healthy individuals. However, in the present study, a trend for higher ARG level in HD patients was detected. Although the reason for the increased levels of these enzymes in HD patients remains to be elucidated, possibly chronic inflammation that characterizes these patients,Citation1 and also detected by increased CRP level in the present study, is responsible. Generally, both IDO and ARG expressions increase in situations that are accompanied by inflammation, such as infection, trauma, or cancer.Citation6–10,12,13,19
Absolute blood T-cell number was significantly lower in HD patients. Interestingly, absolute blood T-cell count was inversely related to plasma IDO and ARG concentrations. Thus, these enzymes with the known immunosuppressive properties could contribute to the impaired acquired immunity that characterizes HD patients, among other mechanisms by decreasing T-cell count. The proapoptotic and antiproliferative effects of IDO and ARG on T-cellsCitation6–11,13,15,17,18 fit with the increased apoptosis, decreased proliferation, and finally, reduced number of T-cells observed in HD patients.Citation20–22
The molecular mechanisms for the immunosuppressive effects of IDO and ARG have been partially elucidated. Tryptophan or arginine depletion in proximity to T-cells leads to intracellular accumulation of deamino acyl transfer RNA (tRNA). Deamino acyl tRNA binds and activates general control nonrepressed 2 (GCN2) kinase, which phosphorylates the eukaryotic translation initiation factor 2 (eIF2). Once GDP-bound eIF2 has been phosphorylated, it is difficult to be converted to GTP-bound form, which is able to translocate the initiator methionine-tRNA to the ribosome. Ultimately, the translation program of lymphocytes alters, with the translation of most but not all proteins to be inhibited.Citation30 The immunosuppressive effects of both tryptophan and arginine depletion on T-cells, are not observed in T-cells derived from GCN2 knockout mice.Citation15,31 In addition, produced by IDO kynurenine pathway products can induce cell cycle arrest and apoptosis in T-cells.Citation6 Furthermore, under suboptimal arginine concentrations due to ARG activity, the reductase and oxygenase domains of nitric-oxide synthase 2 (NOS2) transfer electrons to the co-substrate O2 and produce superoxide, which reacts with other molecules generating reactive nitrogen oxide species and reactive oxygen species. These species can have multiple inhibitory effects on T-cells.Citation32 Thus, IDO and ARG exert their effects on T-cells not only by a shared, well-described molecular pathway, but also by other separate mechanisms. Although no data are available about the effects of ARG on the immune response in HD patients, IDO has been shown to be involved in the increased failure rate of response to vaccination against hepatitis B virus in this population.Citation26
In conclusion, plasma IDO and ARG may contribute to impaired adaptive immunity in HD patients by decreasing blood T-cell number.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Semi Dialysis. 2007;20:440–451.
- Smirnoff M, Patt C, Seckler B, Adler JJ. Tuberculin and anergy skin testing of patients receiving long-term hemodialysis. Chest. 1998;113:25–27.
- Eleftheriadis T, Tsiaga P, Antoniadi G, . The value of serum antilipoarabinomannan antibody detection in the diagnosis of latent tuberculosis in hemodialysis patients. Am J Kidney Dis. 2005;46:706–712.
- Kreft B, Klouche M, Kreft R, Kirchner H, Sack K. Low efficiency of active immunization against diphtheria in chronic hemodialysis patients. Kidney Int. 1997;52:212–216.
- Friedman EA, Beyer MM, Hirsch SR, Schiffman G. Intact antibody response to pneumococcal capsular polysaccharides in uremia and diabetes. JAMA. 1980;244:2310–2311.
- King NJ, Thomas SR. Molecules in focus: Indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol. 2007;39:2167–2172.
- Mulley WR, Nikolic-Paterson DJ. Indoleamine 2,3-dioxygenase in transplantation. Nephrology (Carlton). 2008;13:204–211.
- Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: Focus on hematology. Blood. 2009;113:2394–2401.
- Mellor AL, Munn DH. Ido expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774.
- Mellor AL, Munn DH. Creating immune privilege: Active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80.
- Fallarino F, Grohmann U, Vacca C, . T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077.
- Darcy CJ, Davis JS, Woodberry T, . An observational cohort study of the kynurenine to tryptophan ratio in sepsis: Association with impaired immune and microvascular function. PLoS One. 2011;6:e21185.
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174.
- Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J Biol Chem. 2002;277:21123–21129.
- Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573.
- Eleftheriadis T, Kartsios C, Yiannaki E, . Chronic inflammation and T cell zeta-chain downregulation in hemodialysis patients. Am J Nephrol. 2008;28:152–157.
- Moline-Velazquez V, Cuervo H, Vila-Del Sol V, Ortega MC, Clemente D, de Castro F. Myeloid-derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathol. 2011;21:678–691.
- Liu YY, Sun LC, Wei JJ, . Tumor cell-released TLR4 ligands stimulate Gr-1+CD11b+F4/80+ cells to induce apoptosis of activated T cells. J Immunol. 2010;185:2773–2782.
- Popovic PJ, Zeh III HJ, Ochoa JB. Arginine and immunity. J Nutr. 2007;137:1681S–1686S.
- Ankersmit HJ, Deicher R, Moser B, . Impaired T cell proliferation, increased soluble death-inducing receptors and activation-induced T cell death in patients undergoing hemodialysis. Clin Exp Immunol. 2001;125:142–148.
- Meier P, Dayer E, Blanc E, Wauters JP. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. JASN. 2002;13:204–212.
- Kurz P, Kohler H, Meuer S, Hutteroth T, Meyer zum Buschenfelde KH. Impaired cellular immune responses in chronic renal failure: Evidence for a T cell defect. Kidney Int. 1986;29:1209–1214.
- Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol. 1996;114:107–132.
- Yasuda K, Okuda K, Endo N, . Hypoaminotransferasemia in patients undergoing long-term hemodialysis: Clinical and biochemical appraisal. Gastroenterology. 1995;109:1295–1300.
- Schefold JC, Zeden JP, Fotopoulou C, . Increased indoleamine 2,3-dioxygenase (ido) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: A possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant. 2009;24:1901–1908.
- Eleftheriadis T, Liakopoulos V, Antoniadi G, Stefanidis I, Galaktidou G. Indoleamine 2,3-dioxygenase is increased in hemodialysis patients and affects immune response to hepatitis B vaccination. Vaccine. 2011;29:2242–2247.
- Eleftheriadis T, Antoniadi G, Liakopoulos V, Stefanidis I, Galaktidou G. Plasma indoleamine 2,3-dioxygenase concentration is increased in hemodialysis patients and may contribute to the pathogenesis of coronary heart disease. Ren Fail. 2012;34:68–72.
- Pawlak K, Brzosko S, Mysliwiec M, Pawlak D. Kynurenine, quinolinic acid—The new factors linked to carotid atherosclerosis in patients with end-stage renal disease. Atherosclerosis. 2009;204:561–566.
- Eleftheriadis T, Liakopoulos V, Antoniadi G, Stefanidis I, Galaktidou G. Arginase type I as a marker of coronary heart disease in hemodialysis patients. Int Urol Nephrol. 2011;43:1187–1194.
- Asano K, Sachs MS. Translation factor control of ribosome conformation during start codon selection. Genes Dev. 2007;21:1280–1287.
- Munn DH, Sharma MD, Baban B, . GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642.
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654.