Abstract
Aim: The aim of this study was to investigate the protective effect of caffeic acid phenethyl ester (CAPE) on acetylsalicylic acid (ASA)-induced renal damage in rats. Materials and methods: A total of 40 rats were randomly divided into five groups, with eight rats in each group—group 1: control, not receiving any medication; group 2: ASA (50 mg/kg/day); group 3: ASA (50 mg/kg/day) + CAPE (20 μg/kg/day); group 4: ASA (100 mg/kg/day); and group 5: ASA (100 mg/kg/day) + CAPE (20 μg/kg/day). ASA and CAPE were given via orogastric gavage for 5 days. The total oxidant status (TOS), total antioxidant capacity (TAC), and paraoxonase-1 (PON-1) activity of the blood samples and kidney tissues were determined. Histopathological examinations of the kidneys were performed using light microscopic methods. Results: The TOS level in the serum of rats and kidney tissues given ASA (groups 2 and 4) significantly increased, but the levels of TAC and PON-1 in these tissues significantly decreased in group 4 when compared with the control rats (p < 0.05). The levels of TAC and PON-1 in the kidney tissues increased and the levels of TOS decreased in the CAPE treatment groups (groups 3 and 5) when compared with the rats in the no CAPE treatment groups (groups 2 and 4). The PON-1, TAC, and TOS values reverted to normal levels in group 5 when compared to group 4 (p < 0.05). These results were supported by histopathological observation. Conclusion: Oxidative stress plays an important role in ASA-induced nephrotoxicity, and CAPE may protect against ASA-induced nephrotoxicity in rats.
INTRODUCTION
Acetylsalicylic acid (ASA; aspirin) is a nonselective inhibitor of cyclooxygenase (COX) enzyme that is widely used for its anti-inflammatory, antipyretic, analgesic, and anti-thrombotic effects.Citation1 It is currently one of the most frequently used drugs in the world, and is a systemic agent that has both beneficial and adverse effects throughout the body.Citation2 Ingestion of toxic doses of ASA may result in severe alterations in mental status, gastrointestinal distress, noncardiogenic pulmonary edema, respiratory alkalosis, metabolic acidosis, nephrotoxicity, renal failure, and death. Acute kidney injury can occur with any nonsteroidal anti-inflammatory drugs (NSAIDs).Citation3 ASA can induce two different forms of acute kidney injury: hemodynamically mediated and acute interstitial nephritis, which directly relates to reduction in prostaglandin synthesis.Citation4,5 In this study, we hypothesized that compounds that act as antioxidants may contribute to the improvement of ASA-mediated renal toxicity.
Caffeic acid phenethyl ester (CAPE) is an active component in honeybee propolis extracts and is considered to have medicinal properties. In various studies, it has been used for its antioxidant, anti-inflammatory, anti-carcinogenic, anti-viral, anti-mutagenic, and immunomodulatory activities.Citation6–12 In addition, it has been shown to protect the kidneys from ischemia-reperfusion injury,Citation13 cisplatin-Citation14 and lithium-inducedCitation15 renal damage, diabetic oxidative damage,Citation16 and electromagnetic field and shock wave-induced oxidative stress.Citation17 The current due to the properties of CAPE, a drug may be useful in the prevention of ASA-induced renal pathology.
However, to the best of our knowledge, no study has been published yet as regards the protective effects of CAPE on ASA-induced renal impairment. Therefore, the aim of this study is to investigate the protective effects of CAPE against ASA-induced renal oxidative stress on the biochemical and histopathological levels of rats.
MATERIALS AND METHODS
Animals and Experimental Design
This study was approved by the Dicle University Animal Ethical Committee and was carried out in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals prepared by the Dicle University Animal Ethical Committee.
Female, 3-month-old Wistar albino rats (250 ± 40 g) were obtained from the animal laboratory at Dicle University. All animals were housed under standard conditions at an ambient temperature of 25 ± 2°C and 12/12 h of light–dark cycle in animal cages and were treated in compliance with the National Institutes of Health guidelines. All experimental procedures were in compliance with the animal use regulations of Dicle University Experimental Research Center, Diyarbakır, Turkey. The rats were randomly divided into the following five groups:
Group 1 (n = 8): control group not receiving any medication.
Group 2 (n = 8): only 50 mg ASA administered; ASA tablet was given via orogastric gavage (50 mg/kg/day dose) for 5 days.
Group 3 (n = 8): 50 mg ASA + CAPE administered; ASA tablet was given via orogastric gavage (50 mg/kg/day dose) for 5 days, and concomitant CAPE was given via orogastric gavage (20 μg/kg/day dose) for 5 days.
Group 4 (n = 8): only 100 mg ASA administered; ASA tablet was given via orogastric gavage (100 mg/kg/day dose) for 5 days.
Group 5 (n = 8): 100 mg ASA + CAPE administered; ASA tablet was given via orogastric gavage (100 mg/kg/day dose) for 5 days, and concomitant CAPE was given via orogastric gavage (20 μg/kg/day dose) for 5 days.
Surgical Procedure
After completion of the treatment, all animals were anesthetized with an intramuscular injection of ketamine HCL (50 mg/kg) (Ketalar; Parke-Davis, Karachi, Pakistan) and xylazine (10 mg/kg) peritoneally on the sixth day, and euthanized after surgical anesthesia. Then, 5 cm3 of blood was taken from the cardiac cavities of the rats, and the kidneys were quickly removed and decapsulated. After the blood samples were collected, the kidneys of all rats were excised immediately, decapsulated, and divided longitudinally into two equal sections. One section was used for biochemical analysis, and the other section was stored in 10% formalin for histopathological examination.
The specimens were harvested and stored at −50°C until assayed for total oxidant status (TOS), total antioxidant capacity (TAC), and paraoxonase-1 (PON-1) activities. After the completion of the fixation procedure, the samples underwent routine histological follow-up and were stained with hematoxylin–eosin (H&E) dye in 5-μm sections. The prepared specimens were examined by a specialist pathologist under light microscopy.
Each blood sample was immediately centrifuged at 4000 rpm +4°C for 10 min and then transferred into an Eppendorf tube. These samples were then kept on ice and allowed to deep freeze at −50°C until the end of the experiment. The excised kidney samples were weighed and immediately stored at −50°C. The tissues were perfused with 1.15% ice-cold KCl, minced, and then homogenized in five volumes (w/v) of the same solution. The protein concentration of the tissues was measured by the Lowry method.Citation18 Assays were performed on the supernatant of the homogenate that was prepared at 14,000 rpm for 30 min at +4°C.Citation19 The serum PON-1 activities were measured spectrophotometrically by a modified version of the Eckerson method.Citation20
The TAC of supernatant fractions was evaluated using a novel automated and colorimetric measurement method developed by Erel.Citation21 The TAC results were expressed as nmol Trolox equivalent/mg protein. The TOS of supernatant fractions was evaluated using a novel automated and colorimetric measurement method developed by Erel.Citation22 The assay was calibrated with hydrogen peroxide, and the results were expressed in terms of nmol H2O2 equivalent/mg protein.
Histopathological Assessment
The kidneys were stored in 10% formalin solution for 48 h. After routine histological tissue preparation, all specimens were embedded in wax and sectioned into 4–5 μm thicknesses, which were then stained with H&E. For histopathological evaluation, all kidneys were examined under light microscopy (Nikon ECLIPSE 80i, Nikon Corp., Tokyo, Japan) by a pathologist blinded to the study groups. Renal injury of the cortex and medulla, including tubular degeneration, tubular dilatation, and tubular cell swelling, and tubular architectural impairment was semi-quantitatively scored for each rat on 10 slides at ×200 to ×400 magnification according to the following criteria: normal, 0; involvement of ≤10% of the cortex and medulla, 1; involvement of 11–20% of the cortex and medulla, 2; involvement of 21–30% of the cortex and medulla, 3; involvement of >30% of the cortex and medulla, 4.
Statistical Analysis
All data were expressed as mean and standard deviation (±SD). The differences between the groups were evaluated by Kruskal–Wallis variance analysis, followed by Mann–Whitney U-test, with Bonferroni correction for binary comparisons. Pearson’s chi-square test was used to compare kidney pathology grades. p-Values <0.05 were considered to be statistically significant. All data were processed using the SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA) statistical package.
RESULTS
Serum Biochemical Results
The biochemical results of rat serum and kidney tissues are shown in . The ASA treatment (50 and 100 mg) significantly decreased the serum TAC and PON-1 levels and increased the serum TOS levels when compared with the control group. CAPE + ASA 50 and CAPE + ASA 100 caused a significant decrease in the TOS serum levels when compared with ASA 50 and ASA 100 alone (p = 0.002 and p = 0.001, respectively). In addition, CAPE + ASA 50 and CAPE + ASA 100 caused a significant increase in the serum TAC and PON-1 levels when compared with ASA 50 and ASA 100 alone.
Table 1. Oxidant and antioxidant parameters in rat groups (mean ± standard deviation).
Kidney Biochemical Results
There were no statistically significant differences between the CAPE + ASA 50 treatment and ASA 50 alone according to the PON-1, TAC, and TOS levels (p = 0.401, p = 0.834, and p = 0.834, respectively). In the rat kidney tissues, the activities of PON-1 and TAC decreased in the ASA 50 and ASA 100 groups when compared with the control group (); however, statistically significant differences were seen only between the ASA 100 and the control groups (p = 0.003 and p = 0.014, respectively). In addition, the TOS levels significantly increased in the ASA 50 and ASA 100 groups when compared with the control group (p = 0.036, p = 0.005, respectively). The CAPE + ASA 100 treatment caused a significant increase in the PON-1 and TAC levels in the rat kidneys when compared with ASA 100 alone (p = 0.002, p = 0.046, respectively). However, the CAPE + ASA 100 treatment did not cause a decrease in the TOS levels in the kidney when compared with ASA 100 alone.
Kidney Histopathological Analysis
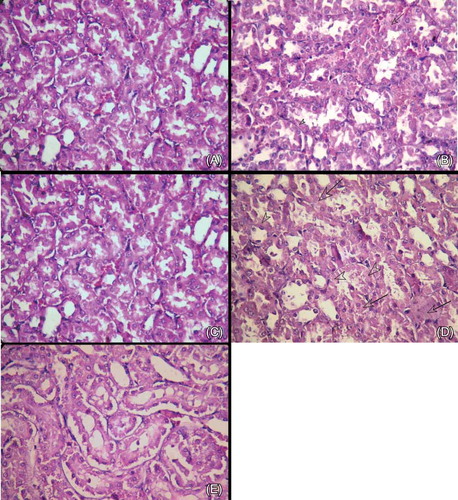
Histopathological evaluation revealed that the renal tissues of the control group had normal structures with no pathological changes (A). The renal sections showed moderate cell swelling (arrow), some nuclear loss (arrowhead), and degenerative changes in the tubular structures in the ASA 50 group (B). Mild tubular cell swelling, some nuclear loss, and minimal degenerative changes in the tubules were observed in the ASA 50 + CAPE group (C). Prominent degenerative changes with cell swelling (arrows), much nuclear loss (arrowheads), nuclear pyknosis in the tubular cells, and moderate architectural impairment in the tubular structures were observed in the ASA 100 group (D). The tubular structure changes, nuclear loss, and cellular swelling decreased with CAPE when compared with ASA 100 alone (E). When the differences among the control group, ASA 50 group, and ASA 100 group were compared, they were statistically different in terms of tubular degeneration and architectural impairment (p < 0.05). When CAPE was added to ASA 50, the improvement was statistically significant (p = 0.032). When CAPE was added to ASA 100, the improvement was higher, but the difference was not statistically significant.
Figure 1. (A) Control group. Renal tubular structures (H&E, ×400). (B) ASA 50 mg group. Moderate cell swelling (arrow), some nuclear loss (arrowhead), and degenerative changes in tubular structures (H&E, ×400). (C) ASA 50 mg + CAPE group. Mild tubular cell swelling, some nuclear loss, and minimal degenerative changes in the tubules (H&E, ×400). (D) ASA 100 mg group. Prominent degenerative changes with cell swelling (arrows), much nuclear loss (arrowheads), nuclear pyknosis in the tubular cells, and moderate architectural impairment in the tubular structures (H&E, ×400). (E). ASA 100 mg + CAPE group. Improvement in the tubular structures, reduction of nuclear loss, and minimal cellular swelling compared with the ASA 100 mg group (Figure 1D) (H&E, ×400).
DISCUSSION
Oxidative stress is the result of an increase in reactive oxygen species (ROS) or an impairment of antioxidant defense systems. Increased ROS attacks cellular structures, including lipids, proteins, and DNA. Various drugs such as ASA may cause oxidative damage in tissues. Lipid peroxidation products are measured as an index of oxygen-free radical generation. The TOS measurements provide a sensitive index of lipid peroxidation and oxidative stress.Citation23 In the kidney tissues and serum, we found that both the 50–mg and 100–mg ASA doses caused a significant increase in the TOS levels, which may be due to overproduction or decreased excretion of oxidant substances. We also found that the TAC levels and PON-1 activities decreased in the ASA-administered rats when compared with the controls. The decrease in the TAC and PON-1 levels may have resulted from overconsumption of all endogenous antioxidants and lipid peroxidation due to ASA-induced nephrotoxicity. These findings support the premise that oxidative stress plays a role in ASA-induced nephrotoxicity.
In this study, we evaluated the protective effects of CAPE in a rat model of aspirin-induced renal impairment. We found that the TOS levels in the serum and kidney tissues of rats in the ASA-administered groups significantly increased, but the levels of TAC and PON-1 in these tissues significantly decreased in the 100-mg ASA-administered group when compared with the control rats. The TAC and PON-1 levels in the serum and kidney tissues of rats in the 50–mg ASA-administered group decreased, but the differences were not statistically significant when compared with the control group. In the CAPE + ASA-administered groups, the TAC and PON-1 levels in the kidney tissues increased and the TOS levels decreased when compared with the rats in the ASA-administered-only groups (no CAPE treatment). The levels of TAC and PON-1 in the serum of rats treated with CAPE increased and the levels of TOS decreased when compared with the rats with no CAPE treatment; however, statistically significant results were seen only between groups 4 and 5. This study demonstrates, for the first time, that CAPE has the ability to reduce oxidative damage caused by ASA-induced oxidative stress in rat serum and kidney tissues. These results are also supported by histopathological observation.
NSAIDs are used in the treatment of various diseases. ASA is predominantly used, in low doses, for the treatment of cardiovascular and cerebrovascular diseases. This type of usage differentiates ASA from other NSAIDs. ASA can induce acute kidney injury by two different forms, which are directly related to the reduction in prostaglandin (PG) synthesis.Citation4,5 Some subtypes of PG provide dilation in vasculature, decrease renal vascular resistance, and increase blood flow to organs. In the intramedullary region of the kidney, this situation leads to the redistribution of blood flow from the renal cortex to the nephrons.Citation24 When an inhibition of these mechanisms occurs, total renal perfusion decreases and the blood flow is redistributed to the cortex, a process that culminates in acute renal vasoconstriction, medullary ischemia, and, under certain conditions, acute renal failure. In patients with decreased renal perfusion, NSAIDs impair renal function; however, those renal complications are reversible through the suppression of NSAIDs. Nonetheless, in the presence of associated adverse conditions, they can cause acute renal dysfunction, nephrotic syndrome, interstitial nephritis, or renal papillary necrosis.Citation25
CAPE and other esters of caffeic acid have anticancerCitation24–26 and anti-inflammatoryCitation10,11,27 properties. The data link these effects of CAPE to inhibition of PG synthesis and, possibly, other products derived from the oxidation of arachidonic acid. CAPE displays anti-inflammatory properties by the suppression of eicosanoid synthesis.Citation27 It is already known that ASA has anti-inflammatory effects. This study shows that CAPE has anti-inflammatory effects. According to the results of this study, CAPE may provide ASA’s anti-inflammatory effects. In the rat model, CAPE caused dose-dependent suppression of PG synthesis and COX-2 in the pouch was markedly suppressed by CAPE.Citation11 CAPE inhibits COX-1 and COX-2 activities and suppresses the release of arachidonic acid from the phospholipids of membrane. The chemo-preventive effects of CAPE can be explained by the known relationship between arachidonic acid metabolism and carcinogenesis. Apoptosis is inhibited by COXs and their end products,Citation28 which could lengthen the survival time of cells with damaged DNA.
CAPE may be useful in the prevention of ASA-induced renal pathology. This is the first study that focused on the protective effects of CAPE on ASA-induced renal injury that was published on PubMed. In this study, the activities of TAC, TOS, and PON-1 were investigated. It was found that CAPE has the ability to reduce ASA-induced oxidative stress damage in rat serum and kidney tissues. Higher TOS levels and decreased TAC levels and PON-1 activities in the ASA-induced rats when compared to the control rats were also found. CAPE pretreatment prevented this increase in TOS and decrease in TAC levels and PON-1 activities in both serum and kidney tissues. Our results show that CAPE has a role in protecting the kidneys from ASA-induced damage.
CONCLUSION
The results suggest that oxidative stress plays an important role in ASA-induced nephrotoxicity. CAPE may provide protection by reducing the concentration of oxidant products and supporting the antioxidant system. Thus, the modulation of oxidative stress with CAPE could be useful in reducing renal impairment caused by aspirin treatment. However, further research is needed to understand the possible mechanisms by which CAPE is able to prevent renal damage.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110(5–6):255–258.
- Alfonso LF, Srivenugopal KS, Bhat GJ. Does aspirin acetylate multiple cellular proteins? (Review). Mol Med Report. 2009;2(4):533–537.
- Schneider V, Levesque LE, Zhang B, Hutchinson T, Brophy JM. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: A population-based, nested case-control analysis. Am J Epidemiol. 2006;164(9):881–889.
- Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3):531–539.
- Murray MD, Brater DC. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol. 1993;33: 435– 465.
- Ek RO, Serter M, Ergin K, . The effects of caffeic acid phenethyl ester (CAPE) on TNBS-induced colitis in ovariectomized rats. Dig Dis Sci. 2008;53(6):1609–1617.
- Marquez N, Sancho R, Macho A, Calzado MA, Fiebich BL, Munoz E. Caffeic acid phenethyl ester inhibits T-cell activation by targeting both nuclear factor of activated T-cells and NF-kappaB transcription factors. J Pharmacol Exp Ther. 2004; 308(3):993–1001.
- Yildiz Y, Serter M, Ek RO, . Protective effects of caffeic acid phenethyl ester on intestinal ischemia-reperfusion injury. Dig Dis Sci. 2009;54(4):738–744.
- Rao CV, Desai D, Kaul B, Amin S, Reddy BS. Effect of caffeic acid esters on carcinogen-induced mutagenicity and human colon adenocarcinoma cell growth. Chem Biol Interact. 1992; 84(3):277–290.
- Sud’ina GF, Mirzoeva OK, Pushkareva MA, Korshunova GA, Sumbatyan NV, Varfolomeev SD. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993;329(1–2):21–24.
- Michaluart P, Masferrer JL, Carothers AM, . Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999;59(10):2347–2352.
- Chen YJ, Shiao MS, Wang SY. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs. 2001;12(2):143–149.
- Ozyurt H, Irmak MK, Akyol O, Sogut S. Caffeic acid phenethyl ester changes the indices of oxidative stress in serum of rats with renal ischemia-reperfusion injury. Cell Biochem Funct. 2001; 19(4):259–263.
- Ozen S, Akyol O, Iraz M, . Role of caffeic acid phenethyl ester, an active component of propolis, against cisplatin-induced nephrotoxicity in rats. J Appl Toxicol. 2004;24(1):27–35.
- Oktem F, Ozguner F, Sulak O, . Lithium-induced renal toxicity in rats: Protection by a novel antioxidant caffeic acid phenethyl ester. Mol Cell Biochem. 2005;277(1–2):109–115.
- Yilmaz HR, Uz E, Yucel N, Altuntas I, Ozcelik N. Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver. J Biochem Mol Toxicol. 2004;18(4):234–238.
- Ozguner F, Oktem F, Ayata A, Koyu A, Yilmaz HR. A novel antioxidant agent caffeic acid phenethyl ester prevents long-term mobile phone exposure-induced renal impairment in rat. Prognostic value of malondialdehyde, N-acetyl-beta-D-glucosaminidase and nitric oxide determination. Mol Cell Biochem. 2005;277(1–2):73–80.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1):265–275.
- Senoglu M, Nacitarhan V, Kurutas EB, . Intraperitoneal Alpha-Lipoic Acid to prevent neural damage after crush injury to the rat sciatic nerve. J Brachial Plex Peripher Nerve Inj. 2009;4:22.
- Eckerson HW, Romson J, Wyte C, La Du BN. The human serum paraoxonase polymorphism: Identification of phenotypes by their response to salts. Am J Hum Genet. 1983;35(2):214–227.
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37(2):112–119.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–1111.
- Uzar E, Alp H, Cevik MU, . Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats. Neurol Sci. 2012;33(3):567–574.
- Rao CV, Desai D, Simi B, Kulkarni N, Amin S, Reddy BS. Inhibitory effect of caffeic acid esters on azoxymethane-induced biochemical changes and aberrant crypt foci formation in rat colon. Cancer Res. 1993;53(18):4182–4188.
- Frenkel K, Wei H, Bhimani R, . Inhibition of tumor promoter-mediated processes in mouse skin and bovine lens by caffeic acid phenethyl ester. Cancer Res. 1993;53(6): 1255–1261.
- Huang MT, Ma W, Yen P, . Inhibitory effects of caffeic acid phenethyl ester (CAPE) on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin and the synthesis of DNA, RNA and protein in HeLa cells. Carcinogenesis. 1996;17(4):761–765.
- Mirzoeva OK, Calder PC. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot Essent Fatty Acids. 1996;55(6): 441–449.
- Lu X, Xie W, Reed D, Bradshaw WS, Simmons DL. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA. 1995;92(17):7961–7965.
