Abstract
A single nodular lesion can be observed in various pulmonary diseases, including cancer, tuberculosis, and fungal infection. Waldenström’s macroglobulinemia (WM) usually occurs in older adults and involves the lymph nodes, bone marrow, and spleen. Respiratory tract involvement is very rare. We reported a case of WM-associated renal amyloidosis. The patient was admitted with the initial presentation as a single mass in the lung and progression to renal involvement.
INTRODUCTION
A single nodular lesion can be observed in various pulmonary diseases, including cancer, tuberculosis, and fungal infection. However, amyloidosis is rare and is not generally considered in the differential diagnosis. Clinical manifestations of localized amyloid deposition within the respiratory tract can be easily confused with pulmonary fibrosis induced as a consequence of chronic respiratory diseases.Citation1,2
Waldenström’s macroglobulinemia (WM) usually occurs in older adults and involves the lymph nodes, bone marrow, and spleen. Respiratory tract involvement is very rare.Citation3 There is often a delay in the definitive diagnosis of WM for slow progression of detectable constructive symptoms.
CASE REPORT
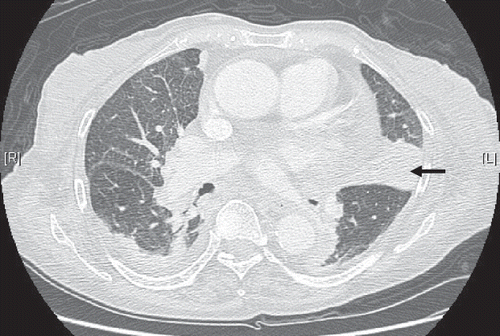
A 77-year-old woman, who was non-smoker, was admitted to our hospital in March 2011 with productive cough, intermittent fever, and progressive shortness of breath. She denied a history of chest pain, weight loss, night sweats, or hemoptysis. There was no significant exposure to toxic agents or animals. Physical examination revealed no palpable lymph node or splenomegaly. Computed tomography (CT) of the chest revealed a solitary small nodule (32 × 30 mm) in the lingual lobe (). CT-guided fine-needle aspiration was performed for suspicious malignancy and pleural seeding. Cytological examination of the specimen taken from the nodule was negative for malignant cells but indicated chronic inflammation. Because pathological examination using CT-guided biopsy did not prove malignancy, additional resection was not performed. Fever and cough subsided after a full course of antibiotic treatment. After 2 weeks of treatment, the patient was asymptomatic and was discharged. In May 2011, follow-up chest radiography showed persisting lower lung shadow.
In August 2011, the patient was readmitted to the hospital with shortness of breath. General weakness and bilateral leg edema had progressed. She denied having fever or bone pain. Laboratory data from blood cell counts and liver function tests were within the normal limit. The serum creatinine level was 1.17 mg/dL. Hypoalbuminemia was also noted. HBV and HCV infection was excluded. Urinalysis revealed heavy proteinuria. Echocardiography indicated normal wall motion and no evidence of dilated cardiomyopathy.
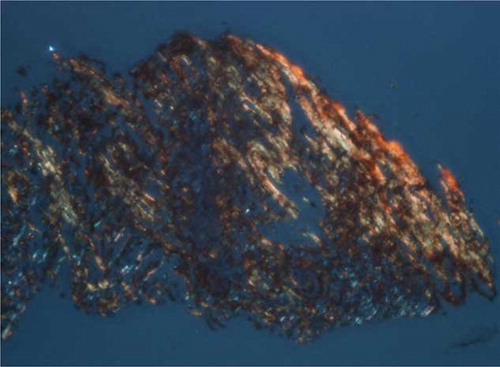
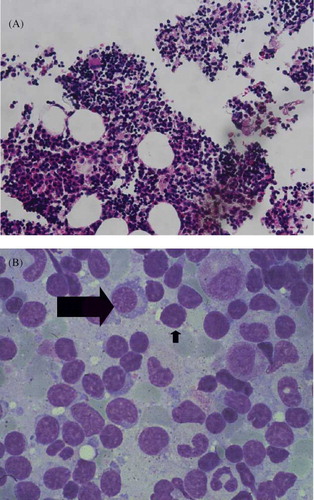
Serum protein electrophoresis showed IgM monoclonal gammopathy at a level of 1470 mg/dL, which exceeded the upper normal limit of 230 mg/dL. The serum viscosity was 1.5 centipoises (cp), which was within the normal range of 1.4–1.8 cp relative to water. Evaluation using urine immunofixation revealed mixed free light chain bands. Congo-red staining of renal biopsy specimens revealed apple-green birefringence under polarized light (). Evaluation of paraffin sections using immunofluorescence demonstrated granular deposition of IgM along vascular walls and in the mesangium. Immunohistochemical staining revealed λ light chain aggregation. We arranged for another CT-guided lung biopsy. Congo-red staining of this biopsy was positive (A and B). Cytokeratin and TTF-1 immunophenotyping was negative for malignancy. Immunohistochemical analysis of biopsied lung and kidney tissues revealed that all amyloid deposits were specifically immunolabeled by an anti-Aλ antibody. The bone marrow study (A and B) revealed expansion of predominantly mature B-lymphocytes with variable plasmacytoid differentiation, and CD5+, CD10−, and CD23−. The proportion of plasma cells was 0.4%. The patient had monoclonal protein in the serum and urine, and there was no evidence of myeloma or change to plasmacytoma.
Figure 2. Renal biopsy.
Note: Congo-red stain reveals apple-green birefringence under polarized light.
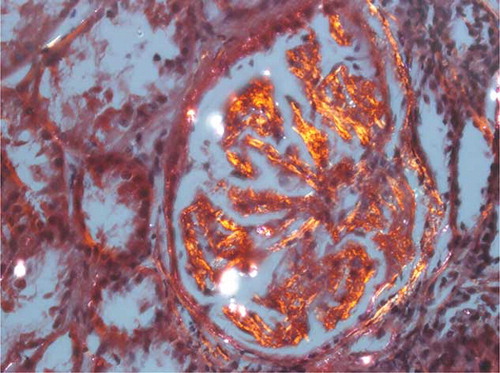
Figure 4. Bone marrow biopsy (A) predominantly mature B-lymphocytes in myeloid (hematoxylin and eosin stain, magnification ×400). (B) Increased aggregation of lymphocytes (short arrow) and plasma cells (long arrow).
The patient was diagnosed as presenting with WM, and systemic AL amyloidosis and nephrotic syndrome. We prescribed prednisolone as the first medication. Chemotherapy with combination therapy including alkylating agents and vinca-alkaloids was not prescribed due to the poor performance status of the patient.
DISCUSSION
WM was first described by Jan G. Waldenström in 1944, who reported two patients with hypergammaglobulinemia, bleeding tendency with oronasal bleeding, anemia, lymphadenopathy, elevated sedimentation rate, hyperviscosity, and cytopenias. There was normal bone films and bone marrow with a predominantly lymphoid infiltration.Citation4 The 2001 World Health Organization (WHO) classification defines WM as a lymphoma of small lymphocytes with variable plasmacytoid differentiation that involves bone marrow and is associated with IgM monoclonal gammopathy. Immunophenotypic studies showed B-lymphocyte antigens positive such as CD19, CD20, and CD22, and usually negative for CD5, CD10, and CD23.Citation3
WM usually occurs in older adults above 70 years of age. There is a higher prevalence of this lesion in men than in women, with involvement of the bone marrow, lymph nodes, and spleen.Citation5 Generally, extranodal involvement and the development of a leukemic phase are rare.Citation3,5
The formation of soluble plasma proteins into an abnormal fibrillar form causes damage to tissue structures and progressive organ dysfunction.Citation6 Amyloidosis affecting lung parenchyma generally appears as patterns of multiple solitary lesions and diffuses alveolar septal involvement.Citation2,7 Characteristic apple-green birefringence due to Congo-red staining was evident under polarized light. Monoclonal IgM can result in several complications when it circulates, accumulates in different tissues, or is associated with autoantibody activity.Citation8 IgM monoclonal immunoglobulin deposition disease accounts for ∼15–20% of monoclonal gammopathy of undetermined significance (MGUS) cases.Citation8 This disease has the potential to progress to smoldering WM and symptomatic WM, and less often to lymphoma or AL amyloidosis.Citation3,8 Rarely, patients with WM (3–5%) have lung involvement, such as diffuse nodules, pulmonary infiltration, masses, or pleural effusion.Citation3 Some WM patients can develop light-chain-associated amyloidosis, with higher incidences of pleural and pulmonary involvement.Citation2,9 Kyrtsonis et al. reported two patients with IgM paraproteinemia with initial presentation of primary lung lymphoma of the lymphoplasmacytic type, without bone marrow involvement. The images of the patients revealed bilateral formation of bilateral consolidation nodules.Citation10 Okuda et al. reported that primary pulmonary WM without systemic secondary involvement is extremely rare and only eight cases have been reported in the English literature review thus far.Citation11 Future studies of amyloid deposition in cases reported as primary pulmonary lesions of WM and the immunological characteristics of cases of amyloidosis may provide a new conceptive conclusion for clinical entities. The observation of microenvironment between cells and other local factors in the lung may provide a brand new clarification of this disease.
WM is incurable with conventional treatments. The overall median survival is only 5–6 years and a median disease-specific survival is 11.2 years.Citation12 The factors associated with poor prognosis include advanced age (>60 years), raised serum glutamic-oxaloacetic transaminase (SGOT) (>41 u/L), high-serum β2-microglobulin (β2M), cytopenias, low albumin level (<35 g/L), serum IgM monoclonal protein, and organomegaly.Citation3,12 The “watch and wait” policy is the standard treatment approach for WM in patients without anemia, systemic symptoms, significant hepatosplenomegaly, bulky lesions, or hyperviscosity.Citation3 A standard treatment for patients with symptomatic or progressive disease has not been yet established.Citation13 First-line therapy of WM was based on single-agent or combination therapy with alkylating agents (e.g., chlorambucil or cyclophosphamide) and nucleoside analogs (cladribine or fludarabine). Monoclonal antibody rituximab has been reported as efficacious in WM. New therapeutic agents that have demonstrated efficacy in WM include lenalidomide, thalidomide, bortezomib, everolimus, atacicept, and perifosine.Citation12,13 The range of the overall response rate to these agents is between 25% and 80%.Citation12 Future clinical trials include PKC inhibitors such as enzastaurin, new proteasome inhibitors such as carfilzomib, histone deacetylase inhibitors such as panobinostat, humanized CD20 antibodies such as ofatumumab, and additional alkylating agents such as bendamustine.Citation12,14 In comparison with traditional chemotherapeutic agents, the new agents may lead to higher responses, longer remissions, and better quality of life for patients with WM.
CONCLUSIONS
Clinical manifestations of localized amyloid deposition within the respiratory tract can be easily confused with those of chronic respiratory diseases such as cancer or tuberculosis. WM with respiratory tract involvement is usually characterized by diffuse nodules, pulmonary infiltration, masses, or pleural effusion. Obtaining tissue samples and evaluating systemic organ involvement are important for diagnosis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Lachmann HJ, Hawkins PN. Amyloidosis and the lung. Chron Respir Dis. 2006;3:203–214.
- Meeus G, Ponette J, Delabie J, Verschakelen J, Blockmans D. Immunoglobulin related amyloidosis presenting as isolated lymph node and pulmonary involvement. Leuk Lymphoma. 2000;38:423–427.
- Vitolo U, Ferreri AJM, Montoto S. Lymphoplasmacytic lymphoma-Waldenstrom’s macroglobulinemia. Crit Rev Oncol Hematol. 2008;67:172–185.
- Waldenström J. Incipient myelomatosis or “essential” hyperglobulinemia with fibrinogenopenia: A new syndrome? Acta Med Scand. 1944;117:216–247.
- Cohen AD, Zhou P, Xiao Q, . Systemic AL amyloidosis due to non-Hodgkin’s lymphoma: An unusual clinicopathologic association. Br J Hematol. 2004;124:309–314.
- Buxbaum JN, Chuba JV, Hellman GC, Solomon A, Gallo GR. Monoclonal immunoglobulin deposition disease: Light chain and light and heavy chain deposition diseases and their relation to light chain amyloidosis. Clinical features, immunopathology, and molecular analysis. Ann Intern Med. 1990; 112:455–464.
- Eguchi T, Yoshida K, Kobayashi N, . Localized nodular amyloidosis of the lung. Gen Thorac Cardiovasc Surg. 2011;59:715–717.
- Kyle RA, Therneau TM, Rajkumar SV, . Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102:3759–3764.
- Stone MJ. Waldenström’s macroglobulinemia: Hyperviscosity syndrome and cryoglobulinemia. Clin Lymphoma Myeloma. 2009;9:97–99.
- Kyrtsonis MC, Angelopoulou MK, Kontopidou FN, . Primary lung involvement in Waldenström’s macroglobulinemia report of two cases and review of the literature. Acta Hematol. 2001;105:92–96.
- Okuda M, Okuda Y, Ogura T, . Primary lung involvement with amyloid deposition in Waldenström’s macroglobulinemia: Observations from over 20 years. Respirology. 2004; 9:414–418.
- Stedman J, Roccaro A, Leleu X, Ghobrial IM. New therapeutic approaches for Waldenstrom macroglobulinemia. Drugs Future. 2010;35:53–58.
- Treon SP. How I treat Waldenström macroglobulinemia. Blood. 2009;114:2375–2385.
- García-Sanz R, Ocio EM. Novel treatment regimens for Waldenström’s macroglobulinemia. Expert Rev Hematol. 2010;3:339–350.