Abstract
Objective: To investigate the protective effect of infliximab on ischemia–reperfusion (I/R) injury of the rat kidney. Methods: Twenty-eight male Wistar albino rats were divided into four groups: sham-operated, I/R, I/R with infliximab administered before ischemia [I/R + infliximab (bi)], and I/R with infliximab administered before reperfusion [I/R + infliximab (br)]. After a right nephrectomy to produce damage, the left renal vessels were occluded for 60 min, followed by 24-h reperfusion in rats. Changes in the rat kidney were observed by measuring the tissue levels of malondialdehyde (MDA), myeloperoxidase (MPO), glutathione (GSH), and superoxide dismutase (SOD) and by evaluating hematoxylin–eosin (H&E)-stained and periodic acid–Schiff (PAS) sections. Results: The MDA and MPO levels in the I/R group were significantly higher than in the other groups (p < 0.05), and the SOD and GSH levels in the I/R + infliximab (bi) and I/R + infliximab (br) groups were significantly higher than in the I/R group (p < 0.05). However, histological examination revealed that the I/R + infliximab (bi) group and the I/R + infliximab (br) group had significantly fewer tubular changes and interstitial inflammatory cell infiltration than the I/R group. Conclusion: These results show that infliximab may protect against I/R injury in the rat I/R model.
INTRODUCTION
Renal ischemia–reperfusion (I/R) injury is a major problem in urological operations, such as renal transplantation, surgical revascularization of the renal artery, and partial nephrectomy.Citation1–3 I/R injury is clinically important because it results in acute renal failure and high mortality.Citation4 In ischemia, renal blood flow is reduced in the kidney by the occluded vessels that supply oxygen to the kidney. Reperfusion has also been reported to aggravate cell damage.Citation5 Reactive oxygen species (ROS) and proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), play a key role in the pathophysiology of renal I/R injury.Citation6,7 Free radicals trigger the accumulation of leukocytes in the tissues. Activated neutrophils secrete enzymes, such as myeloperoxidase (MPO), and liberate more free radicals.Citation8
Infliximab, a novel immunomodulatory agent, has a high affinity for TNF-α. It is being used for refractory Crohn’ s disease, rheumatoid arthritis, and also ulcerative colitisCitation9,10 Recently, researchers demonstrated using human fibroblasts, endothelial cells, neutrophils, lymphocytes, and epithelial cells that infliximab inhibits functional TNF-α activity in various in vitro bioassays.Citation9 Marioni et al. showed that infliximab has a therapeutic effect in treating corticosteroid-resistant Crohn’s disease via a blockade of TNF-α.Citation9,10 In addition, in a recent clinical trial, infliximab—which is used to manage diseases such as rheumatoid arthritis and Crohn’s disease and dermatologic, chronic ocular and neurological diseases—was indicated as an alternative conservative therapy.Citation11–13
However, no study has investigated the effects of infliximab on I/R damage in rat kidneys. Therefore, this study was undertaken to examine whether infliximab shows a protective effect in experimental rat models with I/R-induced renal damage. We evaluated tissue biochemical analysis and histopathological changes in these I/R rat models.
METHODS
Experimental Groups
In this study, we used 28 male Wistar albino rats weighing 250–300 g. The rats were obtained from the Production Center of Experimental Animals at Inonu University, Malatya, Turkey. Throughout the experimental period, all animals were provided, ad libitum, with tap water and standard rat pellet food. The 28 animals were randomly divided into four groups: (I) sham-operated (n = 7), sham-operated animals underwent right nephrectomy only, but the left renal pedicle was not occluded. Renal I/R (n = 7), I/R with infliximab administration before ischemia [I/R + infliximab (bi)] (n = 7), and I/R with infliximab administration before reperfusion [I/R + infliximab (br)] (n = 7). Renal damage measurements were carried out in seven animals for each group. Infliximab (Remicade, Schering Plough Co., Innihannon, County Cork, Ireland) was administered intraperitoneally at a dose of 5 mg/kg.Citation14
All experimental procedures conform to the National Research Council guidelinesCitation15 for the handling and care of laboratory animals. Moreover, all experimental procedures and protocols used in this investigation were reviewed and approved by the Animal Research Committee of Inonu University.
Anesthesia and Ischemia–Reperfusion Procedure
Anesthesia was induced by intramuscular administration of 50 mg/kg ketamine hydrochloride (Ketalar, Pfizer, Istanbul, Turkey) and 10 mg/kg xylazine (Rompun, Bayer, Istanbul, Turkey) before the operation. Right nephrectomy was performed through dorsolateral incisions in all rats. I/R was induced by the occlusion of the left renal vessels for 60 min, followed by 24-h reperfusion. Sham-operated animals underwent right nephrectomy only; the left renal pedicle was not occluded.
At the end of each in vivo study, the rats were killed and the kidneys were quickly removed, decapsulated, and divided equally into two longitudinal sections. One section was placed in formaldehyde solution for a routine histopathological examination using light microscopy. The other section of the kidney was placed in liquid nitrogen and stored at −70°C until the section was assayed for biochemical evaluation.
Histological Analysis
The kidney tissue was fixed in 10% formalin and was embedded in paraffin. Sections of the tissue were cut into 5 μm, mounted on slides, and stained with hematoxylin–eosin (H&E) and periodic acid–Schiff (PAS). These sections were evaluated for the presence of tubular-glomerular injury and interstitial changes. Renal damage was described in terms of the detachment of tubular epithelial cells, loss of brush borders, tubular necrosis, interstitial congestion, glomerular shrinkage, and increased Bowman’s space. The microscopic score for each tissue was calculated as the sum of the scores given to each criterion. The scores given for each criterion were 0 (absent), 1 (slight), 2 (moderate), and 3 (severe).Citation16 For each specimen, 10 microscopic fields were analyzed under a 20× objective per animal. A histopathologist who was unaware of the animal treatment groups examined the tissue sections using a Leica DFC 280 light microscope (Leica Micros Imaging Solutions Ltd., Cambridge, UK).
Biochemical Analysis
Tissues were homogenized (PCV Kinematica Status Homogenizator) in ice-cold phosphate-buffered saline (pH 7.4). The homogenate was sonified with an ultrasonifier (Bronson Sonifier 450) with three cycles (20 s sonications and 40 s pause on ice). The homogenate was centrifuged (15,000×g, 10 min, 4°C), and cell-free supernatant was immediately subjected to enzyme assay.
Malondialdehyde (MDA) level analysis was performed as described with a minor modification.Citation17 The reaction mixture was prepared by adding 250 μL homogenate into 2 mL reaction solution (15% trichloroacetic acid: 0.375% thiobarbituric acid: 0.25 N HCl. 1:1:1. w/v) and heated at 1000°C for 15 min. The mixture was cooled to room temperature, centrifuged (10,000×g for 10 min), and the absorbance of the supernatant was recorded at 532 nm. 1,1,3,3-Tetramethoxypropane was used as MDA standard.Citation17 The values were expressed as nmol/mg protein in the homogenate. Tissue-associated MPO activity was measured using a procedure similar to that documented by Hillegas et al.Citation18 MPO activity was expressed as U/g tissue. Tissue glutathione (GSH) levels were determined using a modified Ellman method.Citation19 The values were expressed as nmol/mg protein in the homogenate. Superoxide dismutase (SOD) activity in the supernatant fraction was measured using the xanthine oxidase/cytochrome c method.Citation20 The amount of SOD in the extract was determined as U/mg protein, utilizing a commercial SOD as the standard.
Statistical Analysis
Data are expressed as means ± SEM. One-way analysis of variance (ANOVA), followed by the Bonferroni test, was applied to calculate the statistical significance between various groups; when p < 0.05, the difference was considered statistically significant.
RESULTS
Biochemical Results
The MDA, MPO, and GSH levels and the SOD activity in the renal tissue of each experimental group are shown in . Renal I/R increased the MDA levels in rats. The MDA levels in the renal I/R (1.33 ± 0.16 nmol/mg) group were significantly higher than those in the sham-operated group (0.52 ± 0.05 nmol/mg). Administering infliximab in rats before ischemia produced a significant reduction in MDA levels compared with those obtained from the I/R group kidneys. In addition, administering infliximab in rats immediately before reperfusion caused significantly decreased MDA levels. However, this reduction was not statistically significant when compared with the results from the I/R + infliximab (bi) group.
Table 1. Effects of infliximab on MDA, MPO, GSH and SOD levels in rats exposed to renal I/R.
Table 2. The comparison of severity of renal damage among groups.
Similarly, renal I/R increased the MPO levels in rats. The MPO levels in the renal I/R (4.30 ± 0.39 U/mg) group were significantly higher than those in the sham-operated group (0.47 ± 0.07 U/mg). Administering infliximab before ischemia produced a significant reduction in MPO levels compared with the levels obtained from the I/R group kidneys. However, administering infliximab immediately before reperfusion also caused significantly decreased MDA levels. This reduction was not noticeable relative to the I/R + infliximab (bi) group.
I/R injury in the kidney tissue caused a significant decrease in the tissue GSH level compared with the levels in other groups. The GSH levels were significantly lower in the renal I/R group (12.56 ± 0.73 nmol/mg) compared with the sham-operated group (24 ± 2.56 nmol/mg). In the I/R+ infliximab (br) group, the GSH concentration was preserved and was not significantly different from that in the sham-operated group. SOD activity was significantly lower in the kidney tissue of the I/R group than in the kidney tissue of the sham-operated group. However, the group administered infliximab before ischemia showed significantly increased SOD levels in the renal tissue.
Microscopy Results
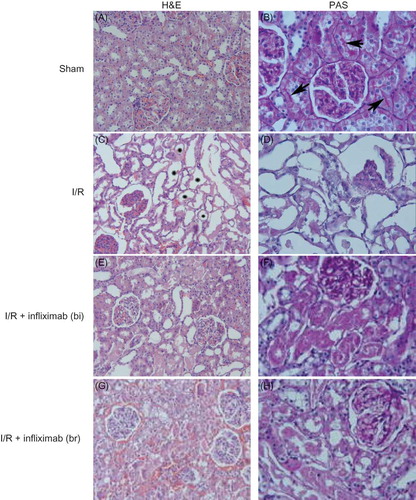
The sham-operated group did not show any detectable histological abnormalities, except slight epithelial desquamation (A and B). However, in the I/R group, extensive cortical and medullar damage was present. Tubular desquamation, tubular necrosis (C), and loss of brush borders (D) were observed in this group. In addition to these changes, most of the glomeruli were damaged. The affected glomeruli had shrunk and Bowman’s space increased (D). Glomerular alterations were detected only in the I/R group. In the I/R, I/R + infliximab (bi), and I/R+ infliximab (br) groups, light microscopic investigation revealed moderate degeneration and tubular necrosis as patchy areas in the renal cortex (E and G). Interstitial congestion was evident in the I/R+ infliximab (br) group (G). Although tubular desquamation and loss of brush borders were present, the glomeruli were intact in these groups (F and H). The results of the semiquantitative histologic analyzes are shown in .
Figure 1. (A) Sham; renal tubules and glomeruli are intact. H&E ×66. (B) Sham; appearance of brush border (arrows). PAS ×132. (C) I/R group; tubules show diffuse and marked necrosis (*). H&E ×66. (D) I/R group; notice lost of the brush border and glomerular changes. PAS ×132. (E) I/R + infliximab (bi); despite tubular degeneration are visible in some area, glomeruli are seen intact. H&E ×66. (F) I/R + infliximab (bi); the loss of brush border was marked in affected tubules. PAS ×132. (G) I/R+ infliximab (br); moderate tubular damage and interstitial congestion are observed. H&E ×66. (H) I/R+ infliximab (br); the view of brush border is similar to ischemia group. PAS ×132.
DISCUSSION
In this study, we found that I/R caused an increase in MDA and MPO levels and a decrease in SOD activity and GSH levels in renal tissue. However, administering infliximab to the I/R group reduced the MDA and MPO values while increasing the SOD activity and GSH levels. I/R caused structural changes in the kidney, but infliximab reversed these changes. These findings indicate that infliximab, along with the TNF-α inhibitor, is important in protecting the kidney from I/R-induced damage in I/R rat models.
After renal surgeries, such as renal transplantation, surgical revascularization of the renal artery, and partial nephrectomy, I/R injury may result from the interruption of the renal arterial circulation.Citation21 As a result, I/R injury may cause acute renal failure. Moreover, ROS play a key role in the pathophysiology of renal IR injury.Citation6,7,22 ROS have many deleterious effects on renal tissue because they react with proteins, membrane lipids, and nucleic acids in the kidney cells.Citation23,24 ROS are generated by mitochondrial cytochrome oxidase, nicotinamide adenine dinucleotide phosphate oxidase, xanthine oxidase, lipoxygenase, cyclooxygenase, hemeoxygenase, cytochrome P-450 enzymes, nitric oxide synthase (NOS), and various other oxidase enzymes. Additionally, ROS may lead to severe injury to the cell membrane by lipid peroxidation reactions. Membrane lipid peroxidation may generate reactive carbonyl compounds such as MDA, one of the reliable indicators of ROS-induced I/R tissue damage.Citation25 MDA is the most abundant aldehyde resulting from lipid peroxidation.Citation26 Among various antioxidant systems equipped within aerobic cells, the key antioxidant enzymes (SOD, GSH) are major mechanisms to reduce local levels of ROS. These enzymes abase primary ROS, such as superoxide anion and hydrogen peroxide, before they can interact to form more reactive cytotoxic metabolites.Citation27 Previous studies have shown that I/R causes an increase in MDA levels and a decrease in GSH levels and SOD activity.Citation28,29 Similarly, we observed a significant decrease in GSH levels and SOD activity and an increase in MDA content during I/R-induced renal injury, making our findings agree with results from previous studies. In our study, infliximab tended to increase SOD activity and GSH levels. These effects were statistically significant. We also observed histopathologic changes in ischemic rat kidneys as well as alterations in oxidative stress markers. These changes are tubular cell swelling, tubular dilatation, cellular vacuolation, medullary congestion, and moderate to severe necrosis.
Proinflammatory factors also play an important role in the pathogenesis of renal I/R injury through leukocyte activation and expression of adhesion molecules and cytokines.Citation14,30,31 Similarly, the increased production of TNF-α has a role in the pathophysiology of I/R injury. MPO, an abundant enzyme in phagocytes, has been implicated in the pathogenesis of various inflammatory diseases.Citation32,33 Thus, MPO is known as an inflammation marker. This enzyme shows an increase in inflammatory conditions. We observed a significant increase in MPO after I/R-induced renal injury.
The literature includes many studies demonstrating that many free radical scavengers and antioxidant agents, such as flavonoids and melatonin, have beneficial effects on I/R-induced damage.Citation23,31,34,35 However, no study has investigated the effects of an immunomodulatory agent, such as infliximab, on I/R damage in rat kidneys. Infliximab, a humanized mouse monoclonal antibody to TNF-α, is a new immunomodulatory agent. Infliximab exerts beneficial effects in several autoimmune diseases, including rheumatoid arthritis, Crohn’s disease, and psoriasis.Citation36 Kurt et al. reported that spinal cord ischemic injury (SCI) reportedly increases MDA levels and that treatment with infliximab decreases the MDA levels in the spinal cord tissue of rats with SCI.Citation37 Furthermore, Guven et al. demonstrated that treatment of I/R injury with infliximab decreased SOD activity and increased GSH levels in spinal cord tissue. In our study, infliximab tended to increase SOD activity and GSH levels. In addition, histologic damage was considerably improved with infliximab treatment.Citation14 Although the results of our study cannot explain the whole mechanism of the attenuation of renal I/R injury, oxidative stress markers and histologic changes suggest that infliximab helps to decrease I/R-induced injury in rat kidneys.
In conclusion, the findings of this study indicate that infliximab has a protective effect on I/R damage in the kidney. Therefore, this agent may be used to prevent renal I/R in clinical practice, particularly in transplantation and renal surgery. However, further studies are necessary to improve our understanding of the role of infliximab in renal I/R.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003; 14(8):2199–2210.
- Akçetin Z, Busch A, Kessler G, . Evidence for only a moderate lipid peroxidation during ischemia-reperfusion of rat kidney due to its high antioxidative capacity. Urol Res. 1999; 27(4):280–284.
- Defraigne JO, Detry O, Pincemail J, . Direct evidence of free radical production after ischemia and reperfusion and protective effect of desferrioxamine: ESR and vitamin E studies. Eur J Vasc Surg. 1994;8(5):537–543.
- Paller MS. Acute renal failure: Controversies, clinical trials, and future directions. Semin Nephrol. 1998;18(5):482–489.
- Weinberg JM. The cell biology of ischemic renal injury. Kidney Int. 1991;39(3):476–500.
- Erdogan H, Fadillioglu E, Emre MH. Protection from renal ischemia reperfusion injury by an endothelin-A receptor antagonist BQ-123 in relation to nitric oxide production. Toxicology. 2006;228(2–3):219–228.
- Serteser M, Koken T, Kahraman A, . Changes in hepatic TNF-alpha levels, antioxidant status, and oxidation products after renal ischemia/reperfusion injury in mice. J Surg Res. 2002;107(2):234–240.
- Sener G, Sehirli O, Velioğlu-Oğünç A, . Montelukast protects against renal ischemia/reperfusion injury in rats. Pharmacol Res. 2006;54(1):65–71.
- Di Sabatino A, Ciccocioppo R, Benazzato L, Sturniolo GC, Corazza GR. Infliximab downregulates basic fibroblast growth factor and vascular endothelial growth factor in Crohn’s disease patients. Aliment Pharmacol Ther. 2004; 19(9):1019–1024.
- Savastano M, Marioni G, Giacomelli L, . Sensorineural hearing loss in ankylosing spondylitis treated with TNF blockers. B-ENT. 2010;6(3):183–188.
- Wildhirt SM, Weismueller S, Schulze C, . Inducible nitric oxide synthase activation after ischemia/reperfusion contributes to myocardial dysfunction and extent of infarct size in rabbits: Evidence for a late phase of nitric oxide-mediated reperfusion injury. Cardiovasc Res. 1999;43(3):698–711.
- Cash D, Beech JS, Rayne RC, . Neuroprotective effect of aminoguanidine on transient focal ischemia in the rat brain. Brain Res. 2001;905(1–2):91–103.
- Vermeulen NP, Baldew GS. The role of lipid peroxidation in the nephrotoxicity of cisplatin. Biochem Pharmacol. 1992; 44(6):1193–1199.
- Guven C, Borcek AO, Cemil B, . Neuroprotective effects of infliximab in experimental spinal cord ischemic injury. J Clin Neurosci. 2010;17(12):1563–1567.
- Gonder JC, Laber K. A renewed look at laboratory rodent housing and management. ILAR J. 2007;48(1):29–36.
- Tasdemir S, Parlakpinar H, Vardi N, Kaya E, Acet A. Effect of endogen-exogenous melatonin and erythropoietin on dinitrobenzene sulfonic acid-induced colitis. Fundam Clin Pharmacol. 2011.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Meth Enzymol. 1978;52:302–310.
- Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24(4):285–295.
- Tok A, Sener E, Albayrak A, . Effect of mirtazapine on oxidative stress created in rat kidneys by ischemia-reperfusion. Ren Fail. 2012;34(1):103–110.
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22):6049–6055.
- Liu F, Lou Y-L, Wu J, . Upregulation of MicroRNA-210 regulates renal angiogenesis mediated by activation of VEGF signaling pathway under ischemia/perfusion injury in vivo and in vitro. Kidney Blood Press Res. 2011;35(3):182–191.
- Sehirli AO, Sener G, Satiroglu H, Ayanoğlu-Dülger G. Protective effect of N-acetylcysteine on renal ischemia/reperfusion injury in the rat. J Nephrol. 2003;16(1):75–80.
- Walker LM, York JL, Imam SZ, . Oxidative stress and reactive nitrogen species generation during renal ischemia. Toxicol Sci. 2001;63(1):143–148.
- Noiri E, Nakao A, Uchida K, . Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281(5):F948–F957.
- Atılgan D, Parlaktas BS, Uluocak N, . Effects of melatonin on partial unilateral ureteral obstruction induced oxidative injury in rat kidney. Urol Ann. 2012;4(2):89–93.
- Elmas O, Elmas O, Caliskan S. Investigation of the oxidative effect of chronic hyperammonemia on the kidney and the possible protective effect of allopurinol. Ren Fail. 2011;33(1):61–65.
- Takahashi M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J Reprod Dev. 2012;58(1):1–9.
- Korkmaz A, Kolankaya D. Protective effect of rutin on the ischemia/reperfusion induced damage in rat kidney. J Surg Res. 2010;164(2):309–315.
- Unal D, Yeni E, Erel O, Bitiren M, Vural H. Antioxidative effects of exogenous nitric oxide versus antioxidant vitamins on renal ischemia reperfusion injury. Urol Res. 2002;30(3): 190–194.
- Xu Y, Liu M, Peng B, . Protective effects of SP600125 on renal ischemia-reperfusion injury in rats. J Surg Res. 2011;169(1):e77–e84.
- Sahna E, Parlakpinar H, Ozturk F, Cigremis Y, Acet A. The protective effects of physiological and pharmacological concentrations of melatonin on renal ischemia-reperfusion injury in rats. Urol Res. 2003;31(3):188–193.
- Wieland E, Brandes A, Armstrong VW, Oellerich M. Oxidative modification of low density lipoproteins by human polymorphonuclear leukocytes. Eur J Clin Chem Clin Biochem. 1993; 31(11):725–731.
- Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94(1):437–444.
- Watanabe M, de Moura Neiva LB, da Costa Santos CX, Martins Laurindo FR, de Fátima Fernandes Vattimo M. Isoflavone and the heme oxygenase system in ischemic acute kidney injury in rats. Food Chem Toxicol. 2007;45(12):2366–2371.
- Sekhon CS, Sekhon BK, Singh I, Orak JK, Singh AK. Attenuation of renal ischemia/reperfusion injury by a triple drug combination therapy. J Nephrol. 2003;16(1):63–74.
- Elliott MJ, Maini RN, Feldmann M, . Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344(8930):1105–1110.
- Kurt G, Ergün E, Cemil B, . Neuroprotective effects of infliximab in experimental spinal cord injury. Surg Neurol. 2009;71(3):332–336, discussion 336.
