Abstract
Since the 2011 nuclear accident in Fukushima, the effects of low-dose irradiation, especially internal exposure, are at the forefront of everyone’s attention. However, low-dose radiation induced various stimulating effects such as activation of antioxidative and immune functions. In this study, we attempted to evaluate the quantitative effects of the activation of antioxidative activities in kidney induced by radon inhalation on carbon tetrachloride (CCl4)-induced renal damage. Mice were subjected to intraperitoneal (i.p.) injection of CCl4 after inhaling approximately 1000 or 2000 Bq/m3 radon for 24 h, or immediately after i.p. injection of α-tocopherol (100, 300, or 500 mg/kg bodyweight). In case of renal function, radon inhalation at a concentration of 2000 Bq/m3 has the inhibitory effects similar to α-tocopherol treatment at a dose of 300–500 mg/kg bodyweight. The activities of superoxide dismutase and catalase in kidneys were significantly higher in mice exposed to radon as compared to mice treated with CCl4 alone. These findings suggest that radon inhalation has an antioxidative effect against CCl4-induced renal damage similar to the antioxidative effects of α-tocopherol due to induction of antioxidative functions.
INTRODUCTION
Carbon tetrachloride (CCl4) is a well-established hepatotoxin.Citation1 A study demonstrated that liver is not the only target organ of CCl4 and it causes free radical generation in other organs, such as the brain, heart, lung, and kidney.Citation2 It has also been reported that CCl4 administration induces oxidative stress in these organs and that vitamin E (α-tocopherol), which is an antioxidant vitamin, inhibits CCl4-induced renal damage.Citation3
A large number of patients are treated in various countries with traditional spa therapy (JapanCitation4–6 and central EuropeCitation7), and Misasa town is especially famous for radon hot spring in Japan. Therapy involving radon gas volatilized from radon-enriched water is performed for treating various diseases at the Misasa Medical Center, Okayama University Hospital. Most conditions treated with radon therapy are pain- or respiratory-related diseases such as arteriosclerosis, osteoarthritis,Citation4 and bronchial asthma.Citation5 Recently, we demonstrated that radon inhalation inhibits CCl4-induced liver and renal damage in mice, indicating that radon inhalation has antioxidative effects in liver and kidney.Citation8 In addition, we demonstrated that radon inhalation has anti-inflammatory effects and inhibits carrageenan-induced inflammatory paw edema.Citation9 Furthermore, in a search for more new indications for radon therapy, we reported the responsiveness of superoxide dismutase (SOD) in mouse organs to radon.Citation10 In that study, we examined the changes in SOD activity in many mouse organs including plasma, brain, lung, thymus, heart, liver, stomach, pancreas, kidney, and small intestine. The results suggest that radon inhalation increases SOD activities in most organs.
Since the 2011 nuclear accident in Fukushima, many reports have been published on the radioactive contaminations in foods and water. Therefore, the effects of low-dose irradiation, especially internal exposure, are at the forefront of everyone’s attention. In contrast, many reports suggest that low-dose irradiation induces various stimulating effects on living organs, especially the activation of biological defense system such as antioxidative and immune functions.Citation11–16 However, there have been no quantitative reports on the antioxidative effects of low-dose irradiation. Therefore, it is difficult for everyone to understand the effects of low-dose irradiation.
The purpose of this study was to compare the antioxidative effects of radon and α-tocopherol. To assess the antioxidative effects of radon, we used the CCl4-induced renal damage model. We examined the following biochemical and histological parameters to assess the effects of radon inhalation on α-tocopherol: creatinine (CRE) level, SOD activity, catalase activity, total glutathione content (t-GSH), lipid peroxide levels, and kidney histology.
MATERIALS AND METHODS
Animals
Female ICR mice (age, 8 weeks; body weight, approximately 28 g) were obtained from Charles River Laboratories Japan Inc. (Yokohama, Japan). Ethical approval for all protocols and experiments was obtained from the animal experimental committee of Okayama University. Mice inhaled radon at a concentration of 1000 or 2000 Bq/m3 for 24 h. The radon concentration in the mouse cage was measured using a radon monitor (CMR-510, Femto-Tech Inc., OH, USA). Mice had free access to food and water during radon inhalation and sham treatment. A total of 4 mL/kg bodyweight of CCl4 (5% in olive oil; Wako Pure Chemical Industries, Ltd., Osaka, Japan) was injected into the peritoneum of the mice immediately after radon inhalation or immediately after (i.p.) injection of DL-α-tocopherol in olive oil (100, 300, or 500 mg/kg weight; Nacalai Tesque Inc., Kyoto Japan). Twenty-four hours after CCl4 administration, blood was drawn from the heart for serum analysis and kidneys were quickly excised to analyze the levels of SOD, catalase, t-GSH, and lipid peroxide. Serum was separated by centrifugation at 3000 × g for 5 min for assay of CRE levels. These samples were preserved at −80°C until biochemical assay. Kidney tissue samples were fixed in 10% neutral buffered formalin for histological examinations.
Biochemical Assays
The CRE level in serum was measured using CRE-EN kainosu (Kainosu Co., Ltd., Tokyo, Japan) according to the manufacturer’s recommendations.
Lipid peroxide levels were assayed using the Bioxytech LPO-586™ assay kit (OXIS Health Products, Inc., Portland, OR, USA) according to the manufacturer’s recommendations. The lipid peroxide assay is based on the reaction between a chromogenic reagent, N-methyl-2-phenylidole, and malondialdehyde and 4-hydroxyalkenals at 45°C. Data were derived from the optical density of the colored products at 586 nm. Briefly, kidney samples were homogenized in 10 mM phosphate-buffered saline (PBS) (pH 7.4) on ice. Prior to homogenization, 10 μL 0.5 M butylated hydroxytoluene in acetonitrile was added per 1 mL of buffer–tissue mixture. After homogenization, the homogenate was centrifuged at 15,000 × g for 10 min at 4°C and the supernatant was used for the assay.
Mouse kidneys were homogenized on ice in 10 mM PBS (pH 7.4). The homogenates were centrifuged at 12,000 × g for 45 min at 4°C and the supernatants were used to assay the activity of SOD and catalase. SOD activity was measured by the nitroblue tetrazolium (NBT) reduction methodCitation17 using the Wako-SOD test (Wako Pure Chemical Industry, Co., Ltd., Osaka, Japan) according to the manufacturer’s recommendations. Briefly, the extent of inhibition of reduction in NBT was measured at 560 nm using a spectrophotometer. One unit of enzyme activity was defined as 50% inhibition of NBT reduction.
Catalase activity was measured as the rate of hydrogen peroxide (H2O2) reduction at 37°C at 240 nm wavelength.Citation18 The assay mixture consisted of 50 μL 1 M Tris–HCl buffer containing 5 mM ethylenediaminetetraacetic acid (pH 7.4), 900 μL 10 mM H2O2, 30 μL deionized water, and 20 μL kidney supernatant. Activity was calculated using a molar extinction coefficient of 0.0071 M−1cm−1.
Total glutathione content was measured using the Bioxytech GSH-420™ assay kit (OXIS Health Products, Inc.) according to the manufacturer’s recommendations. This assay is based on the formation of a chromophoric thione, whose absorbance is at 420 nm and is directly proportional to the total glutathione concentration. Briefly, kidney samples were suspended in 10 mM PBS (pH 7.4), mixed with ice-cold 7.5% trichloroacetic acid solution and homogenized. The homogenates were centrifuged at 3000 × g for 10 min. The supernatants were used for the assay.
The protein content in each sample was measured by the Bradford method, using the Protein Quantification Kit-Rapid (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to the manufacturer’s recommendations.Citation19
Histological Examination
Kidney samples were fixed in 10% formalin, processed through a graded ethanol series and finally xylene, and embedded in paraffin. Six-micrometer-thick tissue sections were prepared and stained with hematoxylin–eosin (H&E). The ratio of Bowman’s capsule in kidney was calculated.
Statistical Analyses
The data values are presented as mean ± 95% confidence intervals. Each experimental group consisted of samples from 5 to 8 animals. The statistical significance of differences was determined by Student’s t-test for comparisons between the control group and CCl4-administrated group. Dunnett’s test was used for multiple comparisons.
RESULTS
Effects of Radon or α-Tocopherol on Renal Function Following CCl4 Administration
To assess the effects of radon inhalation or α-tocopherol treatment on the inhibitory effects of CCl4-induced renal damage, the CRE levels in serum were examined.
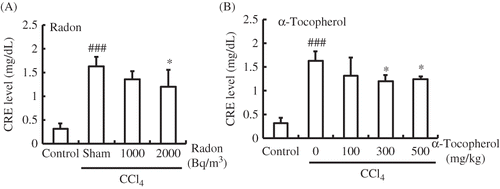
In mice injected with CCl4 in the absence of α-tocopherol or radon pretreatment, the CRE level in serum was significantly higher (p < 0.001) than in control animals. The CRE level in serum of radon-treated mice (2000 Bq/m3; p < 0.05) or α-tocopherol-treated mice (300 or 500 mg/kg weight; p < 0.05) was significantly lower than that of CCl4-administrated mice. Precisely, the CRE levels in serum of radon (1000 or 2000 Bq/m3) or α-tocopherol (100, 300, or 500 mg/kg weight) decreased from 1.63 ± 0.20 to 1.36 ± 0.17, 1.20 ± 0.36, 1.31 ± 0.38, 1.20 ± 0.12, or 1.25 ± 0.06, respectively ().
Figure 1. Effects of radon (A) and α-tocopherol (B) on renal function-associated parameters in the serum of CCl4-administrated mice. Each value indicates the mean ± 95% confidence intervals. The number of mice per experimental point is—six to eight.
Note: *p < 0.05 versus CCl4, ###p < 0.01 versus control.
Effects of Radon or α-Tocopherol on Oxidative Damage Following CCl4 Administration
To assess the inhibitory effects of radon inhalation or α-tocopherol treatment on CCl4-induced renal oxidative damage, the lipid peroxide level in kidney was examined.
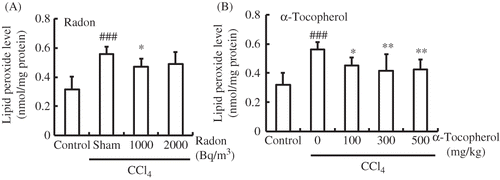
In mice injected with CCl4 in the absence of α-tocopherol or radon pretreatment, the lipid peroxide level in kidney was significantly higher (p < 0.001) than in control animals. However, the lipid peroxide level in the kidney of radon-inhaled mice (1000 Bq/m3) was significantly lower (p < 0.05) in CCl4-administrated mice. In addition, the lipid peroxide level in the kidney of α-tocopherol-treated mice (100, 300, or 500 mg/kg weight) was significantly lower (p < 0.05, p < 0.01, p < 0.01, respectively) than that of CCl4-administrated mice. Precisely, the lipid peroxide levels in kidney of radon (1000 or 2000 Bq/m3) or α-tocopherol (100, 300, or 500 mg/kg weight) decreased from 0.56 ± 0.05 to 0.47 ± 0.06, 0.49 ± 0.08, 0.45 ± 0.05, 0.42 ± 0.12, or 0.42 ± 0.07, respectively ().
Figure 2. Effects of radon (A) and α-tocopherol (B) on oxidative damage-associated parameters in the kidney of CCl4-administrated mice. Each value indicates the mean ± 95% confidence intervals. The number of mice per experimental point is—five to seven.
Note: *p < 0.05, **p < 0.01 versus CCl4, ###p < 0.001 versus control.
Histological Observation in Kidney Following CCl4 Administration
The effects of radon inhalation on the histology of kidneys subjected to CCl4 administration were examined. CCl4 administration resulted in dilatation of Bowman’s space and glomerular atrophy. However, α-tocopherol treatment (100, 300, or 500 mg/kg weight) significantly decreased (p < 0.05, p < 0.05, p < 0.01, respectively) the dilatation of Bowman’s space and glomerular atrophy. Radon inhalation at a concentration of 2000 Bq/m3 inhibited the dilatation of Bowman’s space and glomerular atrophy, but these differences were not statistically significant. Precisely, the Bowman’s space of radon (1000 or 2000 Bq/m3) or α-tocopherol (100, 300, or 500 mg/kg weight) decreased from 0.28 ± 0.02 to by 0.28 ± 0.02, 0.25 ± 0.03, 0.23 ± 0.06, 0.22 ± 0.04, or 0.20 ± 0.03, respectively ().
Figure 3. Effect of radon (H) and α-tocopherol (I) on CCl4-induced renal damage in mouse: (A) control, (B) CCl4, (C) α-tocopherol 100 mg/kg + CCl4, (D) α-tocopherol 300 mg/kg + CCl4, (E) α-tocopherol 500 mg/kg + CCl4, (F) radon 1000 Bq/m3 + CCl4, (G) radon 2000 Bq/m3 + CCl4. Mouse kidneys were examined histologically. The length of the scale bar is 100 μm. All samples were stained with H&E. The arrow indicates dilatation of Bowman’s space with glomerular atrophy. Each value indicates the mean ± 95% confidence intervals. The number of mice per experimental point was—five to six.
Note: *p < 0.05, **p < 0.01 versus CCl4, ###p < 0.001 versus control.
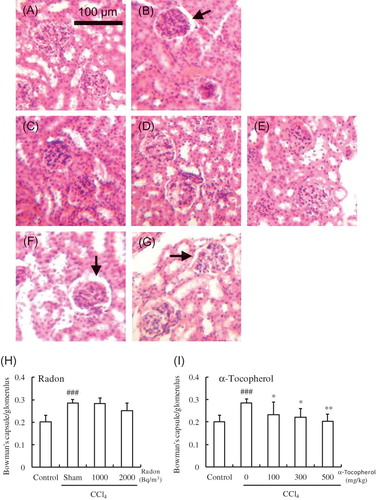
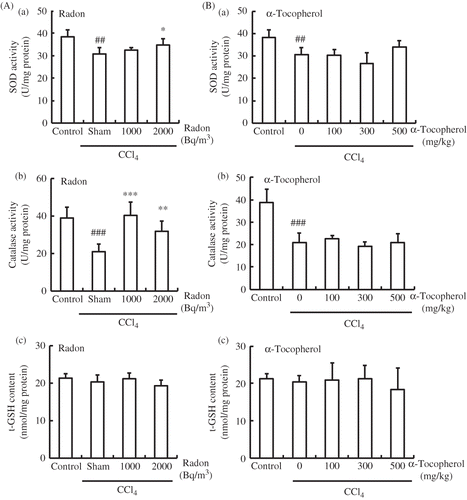
Figure 4. Effects of radon (A) and α-tocopherol (B) on antioxidative-associated parameters in the kidney of CCl4-administrated mice. Each value indicates the mean ± 95% confidence intervals. The number of mice per experimental point is—six to eight.
Note: *p < 0.05, **p < 0.01, ***p < 0.001 versus CCl4, ##p < 0.01, ###p < 0.001 versus control.
Effects of Radon or α-Tocopherol on Antioxidative Functions Following CCl4 Administration
To assess the protective effects of radon inhalation or α-tocopherol treatment on CCl4-induced renal damage, various parameters of oxidative damage were assayed in kidney.
In mice injected with CCl4 in the absence of α-tocopherol or radon pretreatment, the activities of SOD and catalase in kidney were significantly lower (p < 0.01 or p < 0.001, respectively) than in control animals. However, the SOD activities in the kidney of radon-inhaled mice (2000 Bq/m3) and catalase activity in the kidney of radon-inhaled mice (1000 or 2000 Bq/m3) were significantly higher in CCl4-administrated mice. In addition, pre-treatment with α-tocopherol did not result in an increase in SOD or catalase in kidney. Moreover, there were no significant differences in the t-GSH content in kidneys among all groups ().
DISCUSSION
Radon is a radioactive gaseous element that mainly emits α-rays and is a colorless, tasteless, and odorless gas. Therefore, mice are exposed to radon without any stress. In addition, it is easy to estimate the absorbed doses in all organs.Citation20
A report suggested that α-tocopherol administration inhibits CCl4-induced renal damage.Citation3 In contrast, we previously demonstrated that radon inhalation inhibits CCl4-induced renal damage.Citation8 Generally, it is assumed that radiation is harmful to humans. However, we have reported that low-dose irradiation induced various stimulating effects such as activation of antioxidative functions.Citation21–28 In this study, we attempt to compare these inhibitory effects of CCl4-induced renal damage since it is difficult for everyone to understand these radio-adaptive responses that we have already demonstrated.Citation21–28 After the 2011 nuclear accident in Fukushima, the effects of low-dose irradiation are at the forefront of everyone’s attention. Therefore, we conducted this research to give information to everyone about the effects of low-dose irradiation.
The results of this study show that CCl4 administration significantly increases the CRE levels in serum. These findings indicate that CCl4 administration depresses renal function. However, radon inhalation at a concentration of 2000 Bq/m3 and α-tocopherol administration at a dose of 300 and 500 mg/kg weight significantly decreased the CRE levels in serum. These findings suggested that radon inhalation and α-tocopherol administration inhibit CCl4-induced renal damage. Furthermore, this inhibitory effect tended to depend on the dosage of radon or α-tocopherol. In case of renal function, radon inhalation at a concentration of 2000 Bq/m3 has the inhibitory effects similar to α-tocopherol treatment at a dose of 300–500 mg/kg bodyweight.
Our results showed that CCl4 administration significantly increases the lipid peroxide levels in kidney. These findings indicate that CCl4 administration induced oxidative damage in kidney. However, radon inhalation at a concentration of 1000 Bq/m3 and α-tocopherol administration at a dose of 100, 300, and 500 mg/kg weight significantly decreased the lipid peroxide levels in kidney. This inhibitory effect did not depend on the dosage of radon or α-tocopherol unlike the CRE levels. In addition, the protective effect of α-tocopherol on CCl4-induced renal damage was larger than that of radon.
It has been reported that CCl4 administration induced mild dilatation of Bowman’s space with glomerular atrophy.Citation29 In addition, we previously reported that radon inhalation inhibited the dilatation of Bowman’s space and glomerular atrophy. In the present study, radon inhalation at a concentration of 2000 Bq/m3 slightly inhibited the dilatation of Bowman’s space. However, out results showed that the inhibitory effects of α-tocopherol are larger than that of radon inhalation.
It is well known that free radicals are one of the major causes of CCl4-induced renal damage.Citation2,30,31 To clarify the mechanisms underlying the differences between radon and α-tocopherol, we examined antioxidant-associated substances such as SOD, catalase, and t-GSH. Results showed that CCl4 administration significantly decreases the activities of SOD and catalase in kidney. These findings indicate that CCl4 administration depresses antioxidative function. However, the activities of SOD (2000 Bq/m3) and catalase (1000 or 2000 Bq/m3) in kidney of radon-inhaled mice were significantly higher than that of CCl4-treated mice. These findings indicate that radon inhalation activated antioxidative functions. In contrast, there were no significant differences in the activities of SOD and catalase in kidneys between CCl4-administrated group and α-tocopherol-treated groups. These findings suggest that activation of antioxidative function induced by radon inhalation has the same effects of α-tocopherol administration.
In conclusion, radon inhalation has an antioxidative effect against α-tocopherol that is comparable to the treatment with α-tocopherol at a dose of 300–500 mg/kg weight, due to activation of antioxidative functions. In case of lipid peroxidation and tissue damage in kidney, radon inhalation was less effective than α-tocopherol administration.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
REFERENCES
- Recknagel RO, Ghoshal AK. Lipoperoxidation as a vector in carbon tetrachloride hepatotoxicity. Lab Invest. 1966;15: 132–148.
- Ahmad FF, Cowan DL, Sun AY. Detection of free radical formation in various tissues after acute carbon tetrachloride administration in gerbil. Life Sci. 1987;41:2469–2475.
- Aaramoye OA. Comparative effects of vitamin E and Kolaviron (a bioflavonoid from Garcinia kola) on carbon tetrachloride-induced renal oxidative damage in mice. Pak J Biol Sci. 2009;12:1146–1151.
- Yamaoka K, Mitsunobu F, Hanamoto K, Mori S, Tanizaki Y, Sugita K. Study on biologic effects of radon and thermal therapy on osteoarthritis. J Pain. 2004;5:20–25.
- Mitsunobu F, Yamaoka K, Hanamoto K, . Elevation of antioxidant enzymes in the clinical effects of radon and thermal therapy for bronchial asthma. J Radiat Res. 2003;44:95–99.
- Kataoka T, Aoyama Y, Sakoda A, Nakagawa S, Yamaoka K. Basic study on biochemical mechanism of thoron and thermal therapy. Physiol Chem Phys Med NMR. 2006;38:85–92.
- Becker K. One century of radon therapy. Int J Low Radiat. 2004;1:334–357.
- Kataoka T, Nishiyama Y, Toyota T, . Radon inhalation protects mice from carbon-tetrachloride-induced hepatic and renal damage. Inflammation. 2011;34:559–567.
- Kataoka T, Teraoka J, Sakoda A, . Protective effects of radon inhalation on carrageenan-induced inflammatory paw edema in mice. Inflammation. 2012;35:713–722.
- Kataoka T, Sakoda A, Ishimori Y, . Study of the response of superoxide dismutase in mouse organs to radon using a new large-scale facility for exposing small animals to radon. J Radiat Res. 2011;52:775–781.
- Kojima S, Matsuki O, Kinoshita I, Gonzalez TV, Shimura N, Kubodera A. Dose small-dose γ-ray radiation induce endogenous antioxidant potential in vivo? Biol Pharm Bull. 1997; 20:601–604.
- Yamaoka K, Kojima S, Takahashi M, Nomura T, Iriyama K. Change of glutathione peroxidase synthesis along with that of superoxide dismutase synthesis in mice spleen after low-dose X-ray irradiation. Biochem Biophys Acta. 1998; 1381:265–270.
- Yamaoka K, Kojima S, Nomura T. Changes of SOD-like substances in mouse organs after low-dose X-ray irradiation. Physiol Chem Phys Med NMR. 1999; 31:23–28.
- Yamaoka K, Edamatsu R, Mori A. SOD activities and decreased lipid peroxide levels induced by low dose X irradiation in rat organs. Free Radic Biol Med. 1991;11:299–306.
- Kojima S, Nakayama K, Ishida H. Low dose gamma-rays activate immune functions via induction of glutathione and delay tumor growth. J Radiat Res. 2004;45:33–39.
- Ishii K, Yamaoka K, Hosoi Y, Ono T, Sakamoto K. Enhanced mitogen-induced proliferation of rat splenocytes by low-dose whole-body X-irradiation. Physiol Chem Phys Med NMR. 1995;27:17–23.
- Baehner RL, Murrmann SK, Davis J, Johnston RB. The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest. 1975;56:571–576.
- Aebi H, Wyss SR, Scherz B, Gross J. Properties of erythrocyte catalase from homozygotes and heterozygotes for Swiss-type acatalasemia. Biochem Genet. 1976;14:791–807.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Sakoda A, Ishimori Y, Kawabe Y, Kataoka T, Hanamoto K, Yamaoka K. Physiologically-based pharmacokinetic modeling of inhaled radon to calculate absorbed doses in mice, rats and humans. J Nucl Sci Technol. 2010;47:731–738.
- Kataoka T, Yoshimoto M, Nakagawa S, Mizuguchi Y, Taguchi T, Yamaoka K. Basic study on active changes in biological function of mouse liver graft in cold storage after low-dose X-irradiation. J Clin Biochem Nutr. 2009;45:219–226.
- Kataoka T, Sakoda A, Yoshimoto M, . Studies on possibility for alleviation of lifestyle diseases by low-dose irradiation or radon inhalation. Radiat Prot Dosimetry. 2011;146:360–363.
- Yoshimoto M, Kataoka T, Toyota T, Taguchi T, Yamaoka K. Inhibitory effects of prior low-dose X-irradiation on cold induced brain injury in mouse. Inflammation. 2012;35: 89–97.
- Kataoka T, Mizuguchi Y, Yoshimoto M, Taguchi T, Yamaoka K. Inhibitory effects of prior low-dose X-irradiation on ischemia-reperfusion injury in mouse paw. J Radiat Res. 2007;48: 505–513.
- Kataoka T, Yamaoka K. Activation of biodefense system by low-dose irradiation or radon inhalation and its applicable possibility for treatment of diabetes and hepatopathy. ISRN Endocrinol. 2012;2012:1–11.
- Kataoka T, Nomura T, Wang DH, Taguchi T, Yamaoka K. Effects of post low-dose X-ray irradiation on carbon tetrachloride-induced acatalasemic mice liver damage. Physiol Chem Phys Med NMR. 2005;37:109–126.
- Kataoka T, Mizoguchi Y, Notohara K, Taguchi T, Yamaoka K. Histological changes in spleens of radio-sensitive and radio-resistant mice exposed to low-dose X-ray irradiation. Physiol Chem Phys Med NMR. 2006;38:21–29.
- Aoyama Y, Kataoka T, Nakagawa S, . Study on effects of thoron and thermal treatment for aging-related diseases on humans. Iran J Radiat Res. 2012;9:221–229.
- Ogeturka M, Kusa I, Colakoglub N, Zararsiza I, Ilhanc N, Sarsilmaza M. Caffeic acid phenethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J Ethnopharmacol. 2005;97:273–280.
- Ogeturk M, Kus I, Colakoglu N, Zararsiz I, Ilhan N, Sarsilmaz M. Caffeic acid phenyl ester protects kidney against carbon tetrachloride toxicity in rats. J Ethnopharmacol. 2005;97: 273–280.
- Ozturk F, Ucar M, Ozturk IC, Vardi N, Batcioglu K. Carbon tetrachloride-induced and protective effect of betaine in Sprague-Dawley rats. Urology. 2003;62:353–356.
