Abstract
Background: This study investigated the effects of losartan intervention on the expressions of hypoxia-inducible factor-1α (HIF-1α), matrix metalloproteinase-9 (MMP-9), and tissue inhibitor of metalloproteinase-1 (TIMP-1) in renal fibrosis in rats with 5/6 nephrectomy. Methods: Sprague Dawley rats were randomly divided into three groups. Rats in the losartan group were gavaged with losartan (33.3 mg/kg/day) from 1 week after 5/6 nephrectomy, and those in the sham group and the model group only received an equal volume of saline solution by gavage. Rats were sacrificed at the ends of the 4, 8 and 12 weeks, respectively. Urinary N-acetyl-glucosaminidase (NAG), 24-h urinary protein, serum cystatin C, blood urea nitrogen (BUN), and serum creatinine (Scr) levels were assessed. Kidney tissues were observed under light and electron microscope. The expressions of HIF-1α, transforming growth factor-β1 (TGF-β1), MMP-9, and TIMP-1 were determined by immunohistochemistry and Western blotting. Results: Twenty-four hour urinary protein, urinary NAG, serum cystatin C, BUN, and Scr levels in the model group were significantly higher than those in the sham group (p < 0.05), but losartan treatment improved these changes. The apparent glomerular sclerosis and tubulointerstitial fibrosis were also found in the model group, which were ameliorated by losartan. The expressions of HIF-1α, TGF-β1, MMP-9, and TIMP-1 were elevated and MMp-9/TIMP-1 ratio was lowered in the model group (p < 0.05), but losartan increased the expression of MMP-9 and MMp-9/TIMP-1 ratio (p < 0.05) and lessened the expressions of HIF-1α, TGF-β1, and TIMP-1 (p < 0.05). Conclusion: Losartan may ameliorate renal fibrosis partly by down-regulating HIF-1α and up-regulating MMP-9/TIMP-1 in rats with 5/6 nephrectomy.
INTRODUCTION
Chronic kidney disease (CKD) has become a major threat to human health and a global public health issue following cardiovascular and cerebrovascular diseases, cancer, and diabetes. According to data published by the US Renal Data System (USRDS),Citation1 the total treated end-stage renal disease (ESRD) population rose above 570,000 in 2009. The rate of prevalent ESRD cases reached 1738 per million population, an increase of 2.1% in 2008 and consistent with a similar rise per year since 2002.Citation1 It is estimated that in the next 10 years, the number will increase at a rate of 4.1% per year.Citation2 A latest epidemiological study in China showed that the overall prevalence of CKD was 10.8%, and the number of patients with CKD in China is estimated to be about 119.5 million (112.9–125.0 million).Citation3
Renal fibrosis, including glomerulosclerosis and tubulointerstitial fibrosis, is a final common pathway to end-stage renal failure. Once renal damage reaches a certain threshold, the progression of CKD is consistent, irreversible, and largely independent of the initial insults. Chronic hypoxia plays a central pathogenic role in the process of tubulointerstitial injury and ESRD.Citation4 Hypoxia-inducible factor-1α (HIF-1α) stabilization in hypoxic conditions is the critical factor in the development of renal fibrosis and subsequent renal failure.Citation5 Long-term activation of HIF-1α in CKD can regulate multiple signaling pathways to involve in the pathological process of renal fibrosis. HIF-1α not only mediates angiotensin II-(ANG II) induced profibrotic effects by the activation of cell transdifferentiation but also stimulates collagen accumulation by the activation of fibrogenic factors, such as plasminogen activator inhibitor (PAI) and tissue inhibitor of metalloproteinase-1 (TIMP-1). Besides, HIF-1α also contributes to renal epithelial cell collagen expression and renal fibrosis by a talk with transforming growth factor-β (TGF-β)/Smad3 signaling.Citation6
ANG II is a major pathogenic factor producing renal fibrosis in chronic renal injury. ANG II inhibits HIF prolyl-hydroxylases activity and increase HIF-1α level, indicating a possible role of HIF prolyl-hydroxylases in ANG II-induced profibrotic action.Citation7 It has been well recognized that ANG II AT1 receptor blocker (ARB) can reduce proteinuria and block the progression of CKD, but the detailed mechanisms for ARB to protect the kidneys from chronic damage are not fully understood. Previous studies demonstrated that the ARBs played an anti-fibrosis role through the inhibition of the expression of TGF-β and its downstream factors.Citation8 A recent study indicated that ARBs could attenuate renal interstitial fibrosis by inhibiting HIF-1a pathway and decreasing TIMP-1 level in rats with diabetic nephropathy.Citation9 Therefore, this study was performed to investigate whether losartan could alleviate renal fibrosis by regulating the expression of HIF-1α and the balance of matrix metalloproteinase-9 (MMP-9)/TIMP-1 in rats with 5/6 nephrectomy.
MATERIALS AND METHODS
Materials
Animals
Seventy-two clean-grade healthy male Sprague Dawley (SD) rats with 9–10 weeks of age weighing between 160 and 180 g were provided by Shanghai Xipu’er-bikai Experimental Animal Co., Ltd. Qualified No. 2008001603691, license number: SCXK (Shanghai) 2008–0016.
Experimental Medication
Losartan potassium tablets (100 mg/tablet, Batch number: 100094) were provided by Hangzhou MSD Pharmaceutical Co., Ltd., Hangzhou, PR China. Tablets were dissolved and diluted by saline with a concentration of 3.33 mg losartan/mL of solution. The solution was preserved at 4°C in refrigerator.
The Main Reagents
Rat β-N-acetyl glucosaminidase (NAG) enzyme assay kit was provided by Wuhan EIAab Science Co., Ltd., Wuhan, PR China, and rat cystatin C (Cys-C) quantitative kit was provided by R&D system, Inc., MN, USA. HIF-1α, TGF-β1, MMP-9, TIMP-1 primary antibody, β-actin, and the secondary antibody were provided by Santa Cruz Biotechnology, Inc., CA, USA; Coomassie brilliant blue protein assay kit and bicinchoninic acid (BCA) protein concentration assay kit were provided by Fisher Scientific Co., Pittsburgh, PA, USA.
Methods
Animal Model, Grouping, and Medical Administration
Seventy-two SD rats were randomized into the sham group, the model group, and the losartan group. Rats in the model group and the losartan group underwent 5/6 nephrectomy by selective ligation of renal artery branches followed by contralateral nephrectomy, and those in the sham group underwent abdominal incision and manipulation of both kidneys without excision. The procedures were performed by the same operator. Rats in the losartan group were gavaged with losartan (33.3 mg/kg/day) from 1 week after 5/6 nephrectomy, and those in the sham group and the model group only received an equal daily volume of saline solution (10 mL/kg/day) by gavage. The rats in each group were weighed once every week, and the doses of saline solution and losartan were adjusted accordingly.
Histological Analysis of Kidney Tissue Injury
After routine hematoxylin–eosin staining and Masson staining, the renal tissues were observed under light microscopy. Semiquantitative score was given according to the method of Raij et al.Citation10 Scores based on the degree of glomerular sclerosis are 0–4 points (0: normal glomerulus; 1: mesangial expansion or with a sclerotic area of less than 25%; 2: a sclerotic area of 25%–50%; 3: a sclerotic area of 50%–75%; 4: a sclerotic area over 75%). A total of 50 glomeruli per specimen were observed under light microscope at 200× magnification, and the average number, as the mean glomerulosclerosis index, was calculated as follows: (1 × N1 + 2× N2 + 3 × N3 + 4 × N4)/40, where N1, N2, N3, and N4 indicates the number of 1-point, 2-point, 3-point, and 4-point glomerular damage, respectively. Tubulointerstitial injury score: the presences of tubular dilatation, atrophy, necrosis and degeneration of the epithelial cells, interstitial inflammation, and interstitial fibrosis area were given from 0 point to 3 points (0: no tubule interstitial damage; 1: lesions <25%; 2: lesions of 25%–50%; 3: lesion extends more than 50%). Each specimen was observed in 10 fields under light microscope at 200× magnification, and the average was calculated as the renal tubular interstitial lesion score.
Sample Collection and Biochemical Tests
Four, eight, and twelve weeks after administration, eight rats of each group were randomly chosen and sacrificed. The day prior to sacrifice, rats were placed in metabolic cages, fasting but without water limitation, and the 24-h urinary protein was measured after collection of the urine using Coomassie brilliant blue protein kit assay. Rats were anesthetized with 3% iso-pentobarbital sodium, the abdominal aortic blood samples were collected prior to centrifugation (3000 r/min, 15 min), the serum samples were separated for determination of serum BUN Scr (automatic biochemical analyzer) and Cys-C (ELISA kit assay).
Immunohistochemistry and Image Analysis
The expressions of HIF-1α, TGF-β1, MMP-9, and TIMP-1 were determined using immunohistochemical strept avidin-biotin complex (SABC) method. According to kit instructions, the primary antibody (1:100) was added overnight at 4°C, while no antibody was added to the negative control group; phosphate buffered saline (PBS) was then added before the addition of biotin-conjugated secondary antibody and streptavidin-peroxidase. The sample slices were stained with diaminobenzidine (DAB) and being mildly re-stained with hematoxylin. Routine sample dehydration, hyalinization, and mounting were done before observation under microscope. Six samples in each group were analyzed using MIQAS medical image quantitative analysis system (Motic China Group Co., Ltd., Shenzheng, Guangdong, China), and each sample was observed in randomly selected five positive fields under light microscope at 200× magnification; the immunohistochemical positive index was calculated after analysis of immunohistochemical staining. Immunohistochemical positive index = positive area × OD/total area.
Western Blotting
Tissues were placed on ice after taken from the liquid nitrogen; protein was then added followed by repeated grinding and low-temperature centrifugation; the supernatant was separated and protein concentration was measured using the BCA method. A total of 20 μg of sample protein was added per lane before sodium dodecyl sulfate polyacrylamide gel electrophoresis (Bio-Rad, Hercules, USA) with polyacrylamide gel followed by nitrocellulose membrane. After incubated at room temperature, rabbit anti-mouse antibody (1:200) was added and incubated overnight at 4°C. Horseradish peroxidase (HRP) labeled secondary antibody (IgG) was added and incubated for 1 h, and X-ray film was exposed with the light-emitting material luminescence of the enhanced chemiluminescence reagent in the darkroom before developing and fixing. β-actin was used as internal control.
Statistical Methods
All data analyzed using SPSS 15.0 (SPSS Ltd., Chicago, IL, USA) were presented as . Difference between groups was compared using one-way analysis of variance (ANOVA test), difference with homogenous variance between groups was compared using the LSD test, and p < 0.05 was considered statistically significant.
RESULTS
Urine Protein and NAG Enzyme
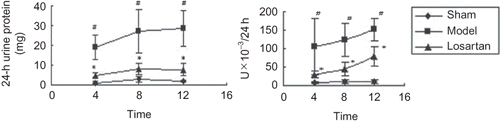
At the ends of 4, 8 and 12 weeks after administration, 24-h urinary protein and NAG excretions in the model group were significantly increased (p < 0.05) when compared with the sham group, whereas losartan treatment reduced significantly the above items (p < 0.05) (see ). The results indicated that urine protein and NAG levels in rats after 5/6 nephrectomy significantly elevated, which were due to glomerular injuries and proximal tubular damages. However, losartan administration reduced urinary protein and NAG enzyme excretion by improving glomerular filter and proximal tubular damages.
Blood Levels of Cys-C, BUN, and Scr
At 4, 8, and 12 weeks, the rats in model group showed a significant increase in serum Cyc-C, BUN, and Scr levels as compared with the sham group (p < 0.05), losartan group demonstrated a reduction in serum Cyc-C and Scr levels(p < 0.05), but did not get a significant decrease in BUN (p > 0.05) (see and ). The results suggested that losartan administration could alleviate renal function impairment induced by 5/6 nephrectomy.
Table 1. Cys-C (mg/L) levels in different groups .
Table 2. BUN and Scr levels in different groups .
Histological Analysis of the Kidney Tissue and Immunohistochemistry
Light Microscopy
The glomerular, tubular, and interstitium structures were normal in the sham group. Four weeks after gavage, glomerular hypertrophy, capsule expansion tubular dilatation, and mesangial cell proliferation with inflammatory cell infiltration in renal interstitium were observed in the model group; at 8 weeks, partial glomerulosclerosis, tubulointerstitial damage, tubule fusion, and necrosis presented; at 12 weeks, focal or complete glomerular sclerosis, renal tubular epithelial cell degeneration and necrosis, partial tubular atrophy, accompanied by some degree of interstitial fibrosis, and renal artery wall thickening in the kidney could be seen; The increasing pathological scores of glomerulosclerosis and tubulointerstitial damages were also found in the model group (see ). However, pathological changes and scores in the losartan group were significant alleviated when compared with the model group in the same period (see ).
Figure 2. Histopathological changes in rat renal tissue samples at 12th week (HE, Hematoxylin–eosin staining ×200; Masson, Masson staining ×200; EM, electron microscopy ×5000); (A) There was no significant histological abnormality in the sham group (HE); (B) glomerular sclerosis, renal tubular epithelial cell degeneration and necrosis, partial tubular atrophy, and some degree of interstitial fibrosis could be seen (HE); (C) in the losartan group, only local mild glomerular and tubular epithelial lesions were observed (HE); (D) there was no fibrosis of the interstitium in the sham group (Masson); (E) glomerular sclerosis with moderate fibrosis of the interstitium was seen in the model group (Masson); (F) a less severe renal fibrosis was observed in the losartan group (Masson); (G) the ultrastructure of intact glomerulus was seen in the sham group (EM); (H) an bending and thickening of the glomerular basement membrane, and podocytes swelling with fusion and flattening of part of the foot processes were found in the model group (EM); (I) less severe damages of the glomerular basement membrane and podocytes were observed in the losartan group (EM); (J) the normal tubule epithelial cells and basement membrane were seen in the control group (EM); (K) in the model group, tubular swelling and degeneration were found with significant interstitial fibroblast proliferation (EM); (L) a Less severe tubulointerstitial damage was observed in the losartan group (EM). Sham: sham-operation group, M: model group, L: losartan group.
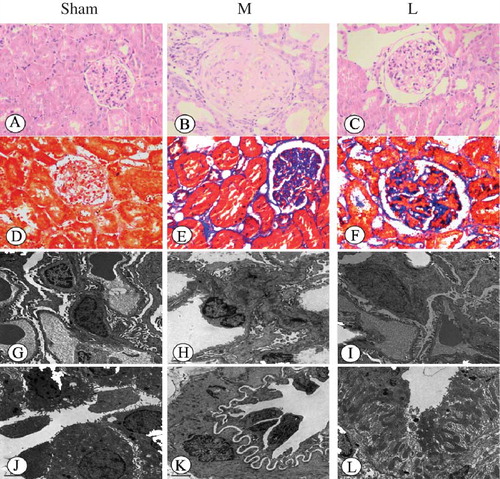
Table 3. Comparison of pathological score of rat kidneys .
Electron Microscopy
Glomerular structure is clear in the sham group with intact filtration membranes and well-shaped epithelial cell foot processes without fusion. Tubular microvilli were found with clear and intact structure with normal endoplasmic reticulum and mitochondria, and the nucleus was approximately round with clear nucleolus. In the model group, the glomerular structure was still clear 4 weeks after gavage, with slightly curved and thickened glomerular basement membrane, showing mesangial cell proliferation, inflammatory response, and increased interstitial fibroblasts; at 8 weeks, swelling of some podocytes, fusion and flattening of part of the of foot processes, and swelling of some tubules and intracytoplasmic vacuoles in some epithelial cells were observed, as well as swelling of microvilli and interstitial fibrosis; at 12 weeks, acceptable glomerular capillary opening, bending and thickening of the basal membrane, podocyte swelling with fusion and flattening of part of the foot processes, and tubular swelling and degeneration were found with significant interstitial fibroblast proliferation. Pathological changes in the losartan group were alleviated compared to the model group in the same period (see ).
Immunohistochemical Results
(1) There was almost no expression of HIF-1α in the glomeruli in the sham group, except a very little expression in the tubulointerstitial tissues, whereas it was abundantly expressed in tubular tissues in the model group (p < 0.05). After losartan treatment, HIF-1α level in renal tissue was significantly decreased when compared with the model group (p < 0.05). (2) There was only tracing tubulointerstitial expression of TGF-β1 in the sham group, whereas it was significantly increased in the model group (p < 0.05). After losartan treatment, TGF-β1 expression in renal tissue was significantly decreased when compared with the model group (p < 0.05). (3) Only tracing glomerular expression of MMP-9 was detected in the sham group, whereas it was significantly increased in the model group (p < 0.01). After losartan treatment, glomerular MMP-9 expression was significantly increased when compared with the model group (p < 0.05). (4) Only a very small amount of renal expression of TIMP-1 was detected in the sham group, whereas it was significantly increased in the model group. After losartan treatment, renal TIMP-1 expression in the model group was significantly decreased (p < 0.05) (see and ). The results demonstrated that losartan treatment caused a significant increase in MMP-9 expression and a significant decrease in TIMP-1expression, indicating that losartan could promote extracellular matrix (ECM) degradation in renal tissue by the up-regulation of MMP-9/TIMP-1.
Figure 3. The expressions of HIF-1α, TGF-β1, MMP-9, and TIMP-1 in rat renal tissues at 12th week (immunochemistry ×200). (1) In sham group, there was a very little expression of HIF-1α in the tubulointerstitial tissues, but abundant expression in tubular tissues in the model group. After losartan treatment, HIF-1α level in renal tissue was lower than in model group (p < 0.05). (2) A tracing tubulointerstitial expression of TGF-β1 was found in the sham group, whereas the expression in the model group increased significantly. Losartan treatment led to significantly decreasing TGF-β1 expression (p < 0.05). (3) Thimbleful glomerular expression of MMP-9 was detected in the sham group, while expression increased in the model group, and losartan treatment further increased significantly glomerular MMP-9 expression (p < 0.05). (4) A very small amount of renal expression of TIMP-1 was detected in the sham group, while expression increased in the model group, and losartan treatment decreased significantly renal TIMP-1 expression (p < 0.05). Sham: sham-operation group, M: model group, L:losartan group.
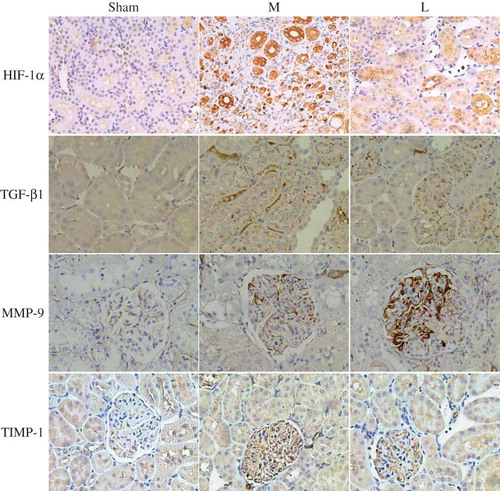
Table 4. Renal expression of HIF-1α, TGF-β1, MMP-9, and TIMP-1 in different groups .
Western Blotting
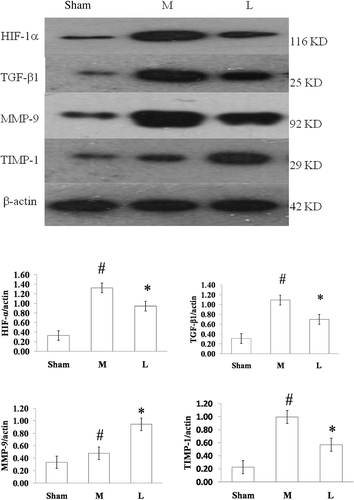
The Western blotting results showed that HIF-1α, TGF-β1 MMP-9, and TIMP-1 protein expressions in the model group were significantly higher than those in the sham group (p < 0.05), and losartan treatment caused a significant reduction of HIF-1α, TGF-β1, and TIMP-1 expressions, but a significant increase of MMP-9 expression (p < 0.05) ().
Figure 4. The difference of HIF-1α (A), TGF-β1 (B), MMP-9 (C), and TIMP-1 (D) protein expressions in rat renal tissues at 12 weeks between different groups. Sham: sham-operation group, M: model group, L: losartan group.
Notes: The expressions of HIF-1α, TGF-β1, MMP-9, and TIMP-1 in the model group were higher than those in the sham group (p < 0.05), losartan treatment induced a decrease in HIF-1α, TIMP-1, and TGF-β1 and an increase in MMP-9 (p < 0.05). #p < 0.05 versus sham-operation group, *p < 0.05 versus model group.
DISCUSSION
Losartan, as a classic drug of ARBs, has been widely recognized to play its protective effects on the kidney by reducing urinary protein, lowering blood pressure, and slowing the progression of chronic renal failure. This study indicated that losartan treatment lowered significantly the serum creatinine (Scr), serum Cyc-C, and 24-h urinary protein in rats with 5/6 nephrectomy(p < 0.05). The glomerulosclerosis and tubulointerstitial fibrosis in rats with 5/6 nephrectomy at 12 weeks were also substantially decreased by losartan treatment. The results indicated that losartan was beneficial to improving renal filter function, reducing urinary protein excretion, and ameliorating renal fibrosis.
Renal fibrosis is characterized by the accumulation of ECM components within the glomerulus and interstitium, which results from the imbalance between ECM production and degradation. Chronic hypoxia is one of the main factors leading to renal ECM accumulation and renal fibrosis, which is mediated by HIF-1α.Citation11,12 Long-term activation of HIF-1α not only mediates ANG II-induced profibrotic effects but also stimulates collagen accumulation by the activation of TIMP-1. The profibrotic effects of ANG II can be attenuated by ARBs through inhibiting HIF-1a pathway and decreasing TIMP-1 level.Citation9
The decreased degradation of ECM is another potential mechanism of renal fibrosis. Normal kidneys produce proteases responsible for the hydrolysis of ECM, among which MMPs are the most important ones, and their activity is subject to the regulation of the inhibitors (TIMPs). MMP-9 is one of the most important MMPs in the human body inducing enzymatic degradation of ECM molecules, and TIMP-1 is specific for the inhibition of MMP-9. The MMP-9/TIMP-1 ratio regulates the aggregation and degradation of the ECM, which are closely related to renal fibrosis.Citation13 A normal ratio of MMP-9 and TIMP-1 plays an important role in the regulation of ECM secretion and accumulation in glomerular mesangial cells.Citation14 ARBs treatment can increase the MMP-9/TIMP-1 mRNA ratio, thereby promoting ECM degradation and ameliorating renal fibrosis.Citation14
Chronic hypoxia can increase the synthesis of ECM, whereas inhibits its degradation, thus causing an accumulation of ECM, the mechanism of which might be hypoxia-induced low expression of MMPs and high expression of TIMPs. The activation of HIF-1α-dependent HGF-signaling can promote the expression of TIMP-1.Citation15 MMP-9/TIMP-1 ratio imbalance, by either reduced MMP-9 expression or increased TIMP-1 expression, promotes the progression of renal fibrosis. However, ARBs treatment not only corrects the MMP-9/TIMP-1 ratio imbalance but also inhibits the activation of HIF-1α. According to these above results, we deduced that the inhibition of renin–angiotensin system (RAS) with ARBs might have a beneficial effect on ameliorating renal fibrosis by regulating HIF-1α level and MMP-9/TIMP-1 ratio balance. This study showed that HIF-1α and TGF-β1 protein expressions in renal tissue in rats with 5/6 nephrectomy were significantly increased after a 12-week period (p < 0.05). Expressions of the two proteins were significantly reduced by losartan treatment (p < 0.05). The results indicate that losartan can reduce the production of ECM by under-regulating HIF-1α expression. Besides, losartan increased MMP-9 expression and decreased TIMP-1 expression in renal tissue in rats with 5/6 nephrectomy, thereby up-regulating MMP-9/TIMP-1 ratio. The results indicate that losartan can promote the degradation of ECM and delay renal fibrosis by improving MMP-9/TIMP-1 ratio balance.
The determinative role of the activation of RAS in the progression of renal fibrosis has been generally accepted. ANG II is the main effective molecule in the RAS system.Citation16 In a hypoxic environment, the renal tissue can stimulate the production of multiple cytokines, which further activate the RAS system and the activity of endothelial cell angiotensin-converting enzymes and increase the synthesis of rennin and angiotensin. ANG II promotes renal fibrosis through pathways such as activation of HIF-1α in renal cells,Citation17 acceleration of fibroblast phenotypic changes, inflammation, and TGF-β1 secretion and increases ECM proteins synthesis through renal interstitial fibroblasts and tubular cell proliferation and hypertrophy by TGF-β1; TGF-β1 can directly increase ECM collagen synthesis from renal tubular cells and interstitial fibrocytes, and it inhibits MMPs activity while facilitates expression of TIMP and PAI-1 genes, thereby inhibiting the degradation of ECM. Our findings demonstrated that losartan could ameliorate renal fibrosis by inhibiting the activation of HIF-1α and regulating MMP-9/TIMP-1 balance, independent of blocking the ANG II-induced effects such as vasoconstriction, sympathetic activation, and secretion of aldosterone.Citation18,19
In summary, losartan has obvious renoprotective effects on rats with 5/6 subtotal nephrectomy. The potential mechanisms may be associated with the decreased production of ECM by down-regulating HIF-1α and the increased degradation of ECM by up-regulating MMP-9/TIMP-1.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
REFERENCES
- Renal Data US, System USRDS. 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
- Gilbertson DT, Liu J, Xue JL, . Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol. 2005;16(12):2753–2758.
- Zhang L, Wang F, Wang L, . Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822.
- Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25.
- Kimura K, Iwano M, Higgins DF, . Stable expression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295(4):F1023–F1029.
- Basu RK, Hubchak S, Hayashida T, . Interdependence of HIF-1α and TGF-β/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. Am J Physiol Renal Physiol. 2011;300(4):F898–F905.
- Page EL, Chan DA, Giaccia AJ, . Hypoxia-inducible factor-1{alpha} stabilization in nonhypoxic conditions: role of oxidation and intracellular ascorbate depletion. Mol Biol Cell. 2008;19:86–94.
- Rüster C, Wolf G. Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. Am Soc Nephrol. 2011;22(7):1189–1199.
- Tang L, Yi R, Yang B, . Valsartan inhibited HIF-1α pathway and attenuated renal interstitial fibrosis in streptozotocin-diabetic rats. Diabetes Res Clin Pract. 2012;97(1):125–131.
- Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26(2):137–143.
- Masaomi N, Reiko I, Toshio M, . Hypoxia and hypoxia-inducible factor in renal disease. Nephron Exp Nephrol. 2008;110(1):e1–e7.
- Li X, Kimura H, Hirota K, . Synergistic effect of hypoxia and TNF-alpha on Production of PAI-l in human proximal renal tubular cells. Kindey Int. 2005;68(2):569–583.
- Kuroda T, Yoshida Y, Kamiie J, . Expression of MMP-9 in mesangial cells and its changes in anti-GBM glomerulonephritis inWKY rats. Clin Exp Nephrol. 2004;8(3):206–215.
- Ding HL, Guo Y, Xu MT, . Effect of angiotensin II receptor blocker on glucoseinduced mRNA expressions of martrix metallopromteinase-9 and tissue inhibitor of metall oproteinase-1 in rat mesangial cells. Chin Med J. 2007;120(21):1886–1889.
- Schelter F, Halbgewachs B, Baumler P, . Tissue inhibitor of metalloproteinases-1-induced scattered liver metastasis is mediated by hypoxia-inducible factor-1α. Clin Exp Metastasis. 2011;28(2):91–99.
- Marumo T, Hishikawa K, Matsuzaki Y, . Angiotensin II type 1 receptor blockade prevents decrease in adult stem like cells in kidney after ureteral obstruction. Eur J Pharmacol. 2007;573:216–220.
- Wang Z, Tang L, Zhu Q, et al. Hypoxia-inducible factor-1α contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int. 2011;79(3):300–310.
- Kong X, Zhang DY, Wu HB, et al. Losartan and pioglitazone ameliorate nephropathy in experimental metabolic syndrome rats. Biol Pharm Bull. 2011;34(5):693–699.
- Meier P, Maillard MP. Combining blockers of the renin-angiotensin system or increasing the dose of an angiotensin II receptor antagonist in proteinuric patients: a randomized triple-crossover study. Hypertension. 2011;29(6):1228–1235.