Abstract
Currently, obesity has reached an epidemic stage and represents a challenge for health authorities across the globe. Certainly, with emergence of obesity epidemic, we started to see an increase in the prevalence of chronic kidney disease (CKD) and nephrolithiasis. Interestingly, epidemiologic studies have shown that the incident stone risk increases with body mass index (BMI), and no further increase in risk is noticed when the BMI > 30 kg/m2. Furthermore, metabolic syndrome and diabetes are also associated with an increase in the incidence of renal stones disease. The shared links between these metabolic disorders are insulin resistance. Furthermore, insulin resistance is thought to alter renal acid–base metabolism, resulting in a lower urine pH and increased risk of uric acid stone disease. Obesity is also associated with excess nutritional intake of lithogenic substances such as refined sugars, low fluid intake, calcium, oxalate, and purine-rich foods. Obesity is also associated with an increase in incidence of urinary tract infection. Recent reports suggested that renal stone disease carries risk of myocardial infarction, progression of CKD, and diabetes. Alarmingly, orlistat (obesity medication) and bariatric surgery are associated with hyperoxaluria and associated stone formation and even oxalate nephropathy. Certainly, the many health risks of obesity, including nephrolithiasis, will add more burden on urologists and nephrologists. Shockwave lithotripsy, percutaneous nephrolithotomy, and ureteroscopy are all safe procedures in obese individuals. Further research is urgently needed to address the pathophysiology and management of obesity-induced renal stones disease.
INTRODUCTION
Renal stones (nephrolithiasis, calculi) disease is common in developed countries with an annual incidence of 1:1000, a peak of onset in the third decade of life, and an increment in prevalence with age until about 70 years.Citation1 The prevalence of kidney stone disease worldwide is estimated to be around 2% and 20%.Citation2–4 The renal calculi can arise in the renal tubules, collecting system, ureters, or bladder and frequently presenting with painful renal colic or hematuria. However, they can be asymptomatic or large enough, as in case of staghorn calculi, to cause urinary obstruction and chronic kidney disease (CKD) that progress to end-stage renal failure (ESRF) and replacement of the native kidney function by dialysis or renal transplantation. Similarly, they can predispose to urinary tract infections (UTIs), cystitis, pyonephrosis, or chronic pyelonephritis, the latest is associated with increased incidence of progressive CKD and ESRF.Citation5 There are six types of renal calculi, namely calcium oxalate, calcium phosphate, mixed calcium oxalate and calcium phosphate, struvite, urate, and cystine. In the United States, uric acid stones represent 10% of all stones and calcium containing stones represent about 70% of all stones, whereas in the Mediterranean and Middle Eastern Countries, the uric acid stones are predominant with a prevalence of about 75%. Struvite stones, which are also known as “triple stones” as they are composed of magnesium ammonium phosphate, account for 10–25% with a higher incidence in the United Kingdom, and 1–2% of stones consist of cystine.Citation2–4 This reflects the geographical variation in the prevalence of nephrolithiasis distribution. The process of stones formation is a complex and insidious one that can only develop in urine that is oversaturated with the chemical or organic constituents of the specific stone.Citation1,2 Renal stones can be due to a variety of causes that can lead to increased urinary excretion of calcium (hypercalciuria with normal serum calcium as in idiopathic hypercalciuria or hypercalciuria with increased serum calcium as in cases in neoplastic and granulomatous diseases), oxalate (hyperoxaluria; dietary hyperoxaluria, enteric oxaluria which due to malabsorptive disorders, primary hyperoxaluria (PH), and high intake of vitamin C), and uric acid (hyperuricosuria).Citation6,7 Other causes include low urinary pH of less than 5.5, low urine volume due to fluid loss, medications (loopdiuretics, vitamin D, salicylates, Probenecid, and Indinavir), metabolic and inherited disorders (PH and cystine stones), renal tubular acidosis, and anatomical disorders with or without chronic UTI.Citation8,9
Furthermore, renal stone disease is associated with a higher prevalence of chronic diseases [hypertension, stroke, myocardial infarction (MI), and diabetes] and adverse cardiovascular outcomes when compared with the general population.Citation10 For instance, during a mean of 9 years of follow-up, stone formers had a 38% [95% confidence interval (CI): 7–77%] increased risk for MI, which remained at 31% (95% CI: 2–69%) after adjustment for CKD and other co-morbidities (CKD, hypertension, diabetes, obesity, dyslipidemia, gout, alcohol dependence, and tobacco use). The authors concluded that kidney stone formers are at increased risk for MI, and this risk is independent of CKD and other risk factors.Citation11 Furthermore, renal stone disease is associated with a higher prevalence of chronic diseases (hypertension, stroke, MI, and diabetes) and adverse cardiovascular outcomes when compared with the general population.Citation10
Moreover, there is consensus that renal stones disease is associated with significantly increased risk of CKD. A nested case–control study was performed in residents of Olmsted County, MN, USA, who presented with a kidney stone at the Mayo Clinic in 1980–1994 to contrast patients with kidney stones who developed CKD with a group that did not. There were 53 cases and 106 controls with a mean age of 57 years at first stone event and 59% men. In kidney stone patients, cases with CKD were significantly more likely (p < 0.05) than controls to have had a history of diabetes (41.5% vs. 17.0%), hypertension (71.7% vs. 49.1%), frequent UTIs (22.6% vs. 6.6%), struvite stones (7.5% vs. 0%), and allopurinol use (32.1% vs. 4.7%) based on univariate analysis.Citation12 The prevalence of this disease in the United States has increased from 3.8% in the 1970s to 5.2% in the 1990s with estimated annual costs totalling $2 billion.Citation13 Due to epidemic of diabetes and obesity, it is expected that the prevalence of renal stone disease will increase, and this will add more to burden of Nephrologists and Urologists. Alarmingly, stone recurrence is common and it is estimated that 50% of stone formers will have recurrence within 5–10 years.Citation6 Therefore, life style management and weight loss are strongly recommended to combat the epidemic of obesity and metabolic syndrome.
Obese patients unable to achieve significant weight loss with lifestyle changes alone may require drug therapy or surgical treatment. We have recently reviewed the role of medical and surgical treatment of obesity on the renal function.Citation14,15 This review provides up-to-date information about obesity, diabetes, metabolic syndrome, and renal stone disease. This review also summarizes important papers about the effect of obesity on surgical treatment of renal stones disease.
OBESITY AND KIDNEY FUNCTION
Obesity is regarded as modifiable risk factor for renal diseases.Citation16 Obesity is associated with an increase in glomerular filtration rate and effective plasma flow associated with increment in filtration fraction and albumin excretion. Different studies suggested that these are first changes in renal function associated with obesity.Citation17,18 Interestingly, obesity is also associated with an increase in kidney weight.Citation19 Furthermore, obesity-related glomerulopathy is associated with glomerulomegaly and focal segmental glomerulosclerosis.Citation20 The features of the metabolic syndrome (insulin resistance, dysglycemia, dyslipidemia, hypertension, and central obesity) are risk factors not only for cardiovascular disease (CVD) but also for renal disease.Citation21,22 Importantly, obesity may be associated with ESRD, renal stone diseases, diabetes, hypertension, and nephrotic syndrome.Citation23 Interestingly, once body mass index (BMI) is greater than 30 kg/m2, further increases do not appear to significantly increase the risk of renal stone disease.Citation24 Medical treatment of obesity and life style modification are two means in treating obesity in large proportions of obese individuals, especially in primary health care.Citation21 Currently, besides its expensive procedure, bariatric surgery is regarded as a last effective choice in maintaining significant weight loss in morbidly obese individuals. Therefore, anti-obesity medications are widely prescribed before bariatric surgery.Citation25 Oxidative stress appears to be the link between CVD, obesity, diabetes, CKD, and renal stone disease.Citation26
OBESITY AND RENAL STONE DISEASE
Several studies have shown that obesity is also associated with increased risk of renal stone formation.Citation27–29 Lee et al. analyzed a database of patient history, BMI, and serum and urine chemistry for 704 consecutive stone formers (467 first-time stone formers and 247 recurrent stone formers). Obesity was significantly associated with stone episodes (p = 0.043). Obese stone formers excreted increased amounts of sodium, calcium, uric acid, and citrate, whereas their urinary pH was lower compared to non-obese stone formers (p < 0.05). Stone analysis revealed that uric acid stone was significantly more commonly found in the obese patients (p = 0.046). Multivariate Cox regression model stratified by stone episodes revealed that obesity [hazard ratio (HR): 2.572, 95% CI: 1.376–4.807, p = 0.003] was the only strong predictor of stone recurrence in first-time stone formers. No association between obesity and stone recurrence was detected in recurrent stone formers.Citation30
Semins et al.Citation24 reviewed data from a 5-year period (2002–2006) in a national private insurance database to identify subjects diagnosed with or treated for kidney stones. From a dataset of 95,598 patients, gender distribution of the 3257 stone formers was 42.9% male and 57.1% female. Obesity (BMI > 30 kg/m2) was associated with a significantly greater likelihood of being diagnosed with a kidney stone. The association of BMI and a stone removal procedure was significant only for men and women with a BMI between 30 and 45 kg/m2 relative to a BMI less than 25 kg/m2 (p < 0.00).Citation24
Nowfar et al. reviewed weighted data from The Nationwide Inpatient Sample contains data on approximately 20% of hospital stays in the United States. The researcher found that the prevalence of nephrolithiasis was relatively stable: 0.52% (149,302) in 1998 and 0.47% (147,541) in 2003. The prevalence of obesity increased from 3.06% (878,155) to 4.99% (1,575,247). The male:female ratio of patients with stones decreased from 1.6:1 to 1.2:1. Multivariate analysis revealed a statistically significant relationship [odds ratio (OR) = 1.22, 95% CI: 1.20–1.23, p < 0.001] between obesity and urinary tract stones. Concurrently, obese females were more likely to develop stones than non-obese females (OR = 1.35, 95% CI: 1.33–1.37, p < 0.001). However, the association between obesity and stones was weaker in males (OR = 1.04, 95% CI: 1.02–1.06, p < 0.001). Accordingly, the authors concluded that obesity was associated with a significantly increased prevalence of urinary stones, and this relationship was stronger in females than in males.Citation31 In addition, two other studies endorsed the notion that obesity-induced renal stone disease is more prevalent in women than men, and this is most likely due to increase in the prevalence of obesity among women.Citation32,33 The association of renal stone formation with obesity may be due to increases in the intake of lithogenic substances such as refined sugars, low fluid intake, calcium, oxalate, and purine-rich foods.Citation34,35 Importantly, obesity is usually associated with insulin resistance which alters renal acid–base metabolism, resulting in a lower urine pH (caused by decreased ammonia production) and increased risk of uric acid stone disease. Obese stone formers excreted increased amounts of sodium, calcium, and uric acid, while their urinary pH in a 24-h urine sample was decreased compared to non-obese stone formers.Citation36,37 Obesity is known to be associated with an increase in risk of uric acid stones formation.Citation37 Multivariate regression modeling stratified by stone incidence has shown that obesity is the only strong predictor of stone recurrence in first-time stone formers.Citation30 No association between obesity and stone recurrence has been detected in recurrent stone formers, suggesting a different mechanism in this group of patients. Interestingly, weight loss has been advocated not only as a recommendation to modify stone risk factors but also as a treatment for obesity-related glomerulopathy.Citation38 Weight loss has been shown to be associated with improvement in glomerular hemodynamics, insulin sensitivity, and decreased urine albumin excretion.Citation39 It is plausible that weight loss can also adversely affect stone risk. Commonly used low-carbohydrate diets increase the risk of both calcium and uric acid stones. Importantly, bariatric surgery (in particular gastric bypass surgery) has been shown to frequently cause enteric hyperoxaluria with increased risk of stone formation and even oxalate nephropathy.Citation14,15
DIABETES AND METABOLIC SYNDROME AND RENAL STONE DISEASE
Alberti et al.Citation40 define an individual with the metabolic syndrome if has any three of the following five criteria—including elevated waist circumference, elevated triglycerides, reduced high-density lipoprotein-cholesterol levels, elevated blood pressure, and elevated fasting-glucose levels. In this new definition, waist circumference is just one of five criteria that physicians can use when diagnosing the metabolic syndrome (population- and country-specific definitions). Interestingly, West et al.Citation41 showed with an increase in the components of the metabolic syndrome there is an increase in the incidence of renal stone diseases. Additionally, Jeong et al. analyzed data obtained from 34,895 individuals who underwent general health screening tests between January 2006 and December 2006. Of all those screened, 839 (2.4%) had radiologic evidence of kidney stones and metabolic syndrome was diagnosed in 4779 (13.7%). The multivariable-adjusted OR for kidney stones increased with an increasing quintile of waist circumference and systolic/diastolic blood pressure (p < 0.001). Age, sex, hypertension, and metabolic syndrome status were independent risk factors for kidney stones. The presence of metabolic syndrome had an OR of 1.25 (95% CI: 1.03–1.50) for kidney stone prevalence. In participants with hypertension, the OR for the presence of kidney stones was 1.47 (95% CI: 1.25–1.71) compared with that for participants without hypertension after adjustment for other variables. Metabolic syndrome is associated with a significantly increased risk of kidney stone development.Citation42 This may be in part explained by the fact that metabolic syndrome is associated with calcium oxalate crystal deposit.Citation43 Asfar et al.Citation44 hypothesized that insulin resistance is associated with uric acid stone formation and the lack of physiological dipping of blood pressure at night. In addition, metabolic syndrome is also associated with decrease in urinary pH and degree of insulin resistance, which creates favorable condition for stone formation.Citation36,45 Furthermore, one renal manifestation of insulin resistance is low urinary ammonium and pH, and this leads to increased risk of uric acid precipitation despite normouricosuria.Citation46,47
Large number of studies showed a link between renal stone disease and type 2 diabetes mellitus, and the risk of diabetes is marked increased in those individual with renal stone disease. Taylor et al. conducted a cross-sectional study of three large cohorts including over 200,000 participants: the Nurses’ Health Study I (older women), the Nurses’ Health Study II (younger women), and the Health Professionals’ Follow-up Study (men), to assess for relationship of diabetes with renal stone disease. They then prospectively studied the association between diabetes and incident nephrolithiasis over a combined 44 years of follow-up. At baseline, the multivariate relative risk of prevalent stone disease in individuals with diabetes compared to individuals without was 1.38 (95% CI: 1.06–1.79) in older women, 1.67 (95% CI: 1.28–2.20) in younger women, and 1.31 (95% CI: 1.11–1.54) in men. Prospectively, the multivariate relative risk of incident kidney stone formation in participants with diabetes compared to participants without was 1.29 (95% CI: 1.05–1.58) in older women, 1.60 (95% CI: 1.16–2.21) in younger women, and 0.81 (95% CI: 0.59–1.09) in men. The multivariate relative risk of incident diabetes in participants with a history of kidney stones compared to participants without was 1.33 (95% CI: 1.18–1.50) in older women, 1.48 (95% CI: 1.14–1.91) in younger women, and 1.49 (95% CI: 1.29–1.72) in men. Their conclusion is that diabetes is a risk factor for the development of kidney stones.Citation48
Interestingly, stone formers with type II diabetes mellitus excrete significantly greater urinary oxalate and significantly lower urine pH than those without diabetes mellitus. In a retrospective study, univariate analysis showed diabetic patients had significantly greater urine volume than non-diabetic patients (2.5 vs. 2.1 L daily, p = 0.004). Those with diabetes mellitus also excreted less daily potassium (61.1 vs. 68.8 mEq, p = 0.04), phosphate (0.84 vs. 1.0 g, p = 0.002) and creatinine (1405.5 vs. 1562.8 mg, p = 0.03), and had significantly lower daily urine pH (5.78 vs. 6.09, p < 0.001) as well as lower calcium-phosphate super saturation (0.49 vs. 1.20, p < 0.001) than non-diabetic patients. On multivariate analysis, patients with type II diabetes mellitus had significantly lower urine pH (−0.34, 95% CI: −0.48 to −0.21) and significantly greater urinary oxalate (6.43 mg daily, 95% CI: 1.26–11.60) and urine volume (0.38 l daily, 95% CI: 0.13–0.64) when compared to patients without diabetes mellitus.Citation49 Diabetes is also linked with risk for uric acid stone.Citation50 Daudon et al. showed the distribution of the main stone components in a series of 2464 calculi from 272 (11%) patients with type 2 diabetes and 2192 without type 2 diabetes. The proportion of uric acid stones was 35.7% in patients with type 2 diabetes and 11.3% in patients without type 2 diabetes (p < 0.0001). Reciprocally, the proportion of patients with type 2 diabetes was significantly higher among uric acid stone formers than among calcium stone formers (27.8 vs. 6.9%; p < 0.0001). Stepwise regression analysis identified type 2 diabetes as the strongest factor that was independently associated with the risk for uric acid stones (OR = 6.9; 95% CI: 5.5–8.8).Citation51 Therefore, patients with uric acid stones and overweight should be screened for diabetes and components of the metabolic syndrome. Another rare complication associated with diabetes and renal stone disease is emphysematous pyelonephritis (EPN), which is a severe acute necrotizing infection of the renal parenchyma and peri-renal tissue, characterized by gas formation and can be fatal if not diagnosed early. Ninety percentage of cases are seen in association with diabetes mellitus and may be due to renal stone disease. Several case reports showed the link between EPN and diabetes and renal stone disease.Citation52–54
OBESITY AND UTI AND RENAL STONE DISEASE
Obesity was shown to be an independent risk factor for nosocomial infection after trauma.Citation55,56 Obesity is associated with risk of UTI. Semins et al.Citation57 reported that increased BMI appears to be associated with an increased risk for UTI and pyelonephritis. In study by the Prostate Study Group of Australian Society for Urology, obesity was identified as risk factor for UTI.Citation58 In addition to above factors, UTI may be another factor that leads to obesity-induced renal stone disease.
OBESITY SURGERY AND MEDICAL TREATMENT OF OBESITY AND RISK OF RENAL STONE DISEASE
Obese patients unable to achieve significant weight loss with lifestyle changes alone may require drug therapy or bariatric surgery. Clinical trials have shown that orlistat administration may not only lead to weight loss but also protect against type 2 diabetes in around 37%. Orlistat can induce and maintain weight loss, even in patients with comorbid conditions such as hypertension or type 2 diabetes. Above and beyond, orlistat can induce marked weight loss in individuals with CKD. However, in small numbers of individuals, especially those with CKD, orlistat administration may precipitate oxalate nephropathy and renal stone disease.Citation15 Decisively, bariatric surgery is regarded as the only therapy that is effective in maintaining significant weight loss in morbidly obese individuals. Despite the fact that bariatric surgery-induced weight loss is associated with a significant decrease in morbidity and mortality and improvement in renal function, bariatric surgery has recently been shown to be associated with a significant risk of nephrolithiasis. The main risk factor for nephrolithiasis is increased excretion of urinary oxalate.Citation14 Therefore, obesity per se is associated with an increase in risk of renal stone disease and also medical and surgical management of obesity is also associated with renal stone disease.
HOW OBESITY-INDUCED RENAL STONE DISEASE CAN BE A CHALLENGE FOR UROLOGIST?
It is well documented in the literature that obesity is associated with high rate of complications before, during, and after surgery. Surgery options for kidney stones include shockwave lithotripsy (SWL), percutaneous nephrolithotomy (PCNL), ureteroscopy (URS), and major surgery. Aboumarzouk et al. performed a meta-analysis that included seven studies with 131 obese patients (mean BMI of 42.2) (136 renal units) treated with flexible URS for urinary calculi. The mode of fragmentation was pulse dye laser, holmium laser, and combined modality including electro hydraulic lithotripsy and basket retrieval in others. The average stone size was 1.37. The stone free rate was 87.5% after completion of treatment with a ranged follow-up between 3 months and 3.5 years. The mean operative time was 97.1 min (30–275). There was an overall 11.4% complication rate; however, none of the patients needed further monitoring and were treated conservatively. A sub-group analysis of the stones depending on size found that the URS has a higher stone free rate in stones less than 2 cm in size (p = 0.0003). Furthermore, URS has a higher stone free rate when treating ureteric stones compared to renal stones (p = 0.04). Retrograde stone treatment using URS is a safe and efficient modality for treating obese patients with urinary tract calculi with an increased efficiency with smaller stones less than 2 cm in size.Citation59 El-Assmy et al. showed that PCNL in obese and morbidly obese patients yield a stone-free rate that is comparable to that achieved in non-obese patients. Moreover, the complication rate and length of hospital stay are also similar.Citation60 Moreover, PCNL can be performed with excellent stone-free rates and with an acceptable complication risk in the diabetic population. Analysis of the data from 183 (13.7%) patients with diabetes of the 1338 patients undergoing PCNL revealed that patient age (63.1 years), surgical time (90.8 min), and complications (major 2.2%), including need for transfusion (0.5%) and stone-free rate (94.5%), were not significantly different from those observed in nondiabetic cohort. However, the average length of hospital stay was marginally longer in the diabetic group (4.4 days vs. 3.9 days, p = 0.022). Yet again, uric acid stone composition was found to be the most common stone composition among the patients with diabetes in this study (41%).Citation61
SWL is reported to be associated with risk for development of diabetes and hypertension. At 19 years of follow-up, SWL for renal and proximal ureteral stones was associated with the development of diabetes mellitus and hypertension. Diabetes mellitus was related to the number of administered shocks and treatment intensity.Citation62 However, two large studies showed that SWL is not associated with diabetes or hypertension. In a study of 5287 individuals with stone formation and without diabetes, after an average follow-up of 8.7 years, 423 patients (8%) were treated with SWL, and new-onset diabetes had developed in 743 (12%). The diagnosis of diabetes mellitus followed SWL in 77 patients. However, no association was evident between SWL and the development of diabetes before (HR: 0.98, 95% CI: 0.76–1.26) or after (HR: 0.92, 95% CI: 0.71–1.18) SWL, controlling for age, sex, and obesity.Citation63 Furthermore, Sato et al.Citation64 showed that SWL is not associated with hypertension or diabetes. Therefore, SWL is safe procedure and it is plausible to monitor patients for diabetes and hypertension.
Taken together, SWL, PCNL, URS are all safe procedures in obese individuals. Importantly, due to epidemic of diabetes, metabolic syndrome, and obesity, it is possible to suggest that there will be an increase in numbers of individuals with obesity-induced renal stone disease. Obviously, this will add extra burden on urology services with regard to number of procedures performed on daily basis, staff training, and devices. Only urologists across the globe can tell whether it is too late to expand in the training of new generation of urologists.
CONCLUSION
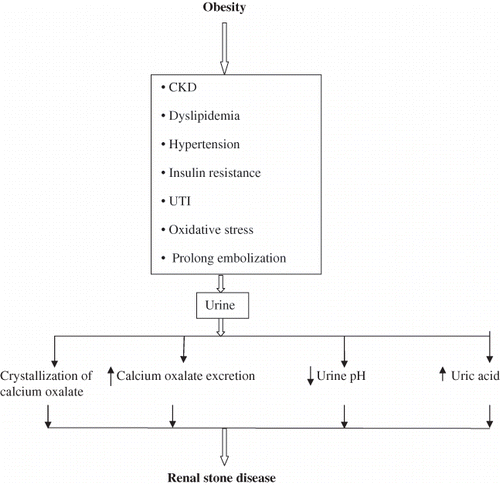
A growing body of evidence that is convincing in balance indicates the relationship between obesity and renal stone disease. Importantly, renal stone disease is associated not only with CKD but also with MI, diabetes, and metabolic syndrome. The underlying mechanism of how obesity is associated with renal stone disease is complex and involves different factors (). Medical and surgical treatment of obesity is associated with increased risk of renal stone disease. Therefore, there is an urgent need for research to address treatment of obesity with less risk for renal stone disease. Importantly, clinical studies are urgently needed to understand the association between renal stone disease and MI and diabetes. Only epidemiologist with special interest in nephrology and urology around the sphere can predict about the need for more manpower in these two specialities in order to tackle the renal complications, including renal stone disease, associated with epidemic of obesity.
Figure 1. Possible mechanism of how obesity may induce renal stone disease.Note: CKD, chronic kidney disease; UTI, urinary tract infection.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Monk RD. Clinical approach to adults. Semin Nephrol. 1996;16:375–388.
- Buchholz NP, Abbas F, Afzal M, Khan R, Rizvi I, Talati J. The prevalence of silent kidney stones – an ultrasonographic screening study. J Pak Med Assoc. 2003;53:24–25.
- Indridason OS, Birgisson S, Edvardsson VO, Sigvaldason H, Sigfusson N, Palsson R. Epidemiology of kidney stones in Iceland: a population-based study. Scand J Urol Nephrol. 2006;40:215–220.
- Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994;46:893–899.
- Hootan TM. Urinary tract infection in adults. In: Johnson RJ, Feehally J, eds. Comprehensive Clinical Nephrology. 2nd ed. London: Mosby; 2003:731–744.
- Ljunghall S, Danielson BG. A prospective study of renal stone recurrences. Br J Urol. 1984;56:122–124.
- Shekarriz B, Stoller ML. Uric acid nephrolithiasis: current concepts and controversies. J Urol. 2002;168:1307–1314.
- McPhail EF, Gettman MT, Patterson DE, Rangel LJ, Krambeck AE. Nephrolithiasis in medullary sponge kidney: evaluation of clinical and metabolic features. Urology. 2012;79:277–281.
- Goldfarb DS, Coe FL. Prevention of recurrent nephrolithiasis. Am Fam Physician. 1999;60:2269–2276.
- Domingos F, Serra A. Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant. 2011;26:864–868.
- Rule AD, Roger VL, Melton LJ III, . Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010;21:1641–1644.
- Saucier NA, Sinha MK, Liang KV, . Risk factors for CKD in persons with kidney stones: a case–control study in Olmsted County, Minnesota. Am J Kidney Dis. 2010;55:61–68.
- Brener ZZ, Winchester JF, Salman H, Bergman M. Nephrolithiasis: evaluation and management. South Med J. 2011;104:133–139.
- Ahmed MH, Byrne CD. Bariatric surgery and renal function: a precarious balance between benefit and harm. Nephrol Dial Transplant. 2010;25:3142–3147.
- Ahmed MH. Orlistat and calcium oxalate crystalluria: an association that needs consideration. Ren Fail. 2010;32:1019–1021.
- Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1565–1574.
- Kasiske BL, Cleary MP, O’Donnell MP, Keane WF. Effects of genetic obesity on renal structure and function in the Zucker rat. J Lab Clin Med. 1985;106:598–604.
- Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211–1217.
- Mathew AV, Okada S, Sharma K. Obesity related kidney disease. Curr Diabetes Rev. 2011;7:41–49.
- Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509.
- Ahmed MH, Byrne CD. Metabolic syndrome, diabetes & CHD risk. In: Packard CJ, ed. The Year in Lipid Disorders. Oxford: Clinical Publishing;2007:3–26.
- Ahmed MH, Khalil A, Osman MM. Nitric oxide as treatment for an emerging epidemic of obesity-related glomerulopathy. Diabetes Res Clin Pract. 2006;74:207–208.
- Wesson DE, Kurtzman NA, Frommer JP. Massive obesity and nephrotic proteinuria with a normal renal biopsy. Nephron. 1985;40:235–237.
- Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R, Matlaga BR. The Association of increasing body mass index and kidney stone disease. J Urol. 2010;183:571–575.
- Ahmed MH, Byrne CD. Current treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2009;11:188–195.
- Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res. 2012;40:95–112.
- Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: a review. Am J Hypertens. 2008;21:257–264.
- Negri AL, Spivacow FR, Del Valle EE, Forrester M, Rosende G, Pinduli I. Role of overweight and obesity on the urinary excretion of promoters and inhibitors of stone formation in stone formers. Urol Res. 2008;36:303–307.
- Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. J Am Med Assoc. 2005;293:455–462.
- Lee SC, Kim YJ, Kim TH, Yun SJ, Lee NK, Kim WJ. Impact of obesity in patients with urolithiasis and its prognostic usefulness in stone recurrence. J Urol. 2008;179:570–574.
- Nowfar S, Palazzi-Churas K, Chang DC, Sur RL. The relationship of obesity and gender prevalence changes in United States inpatient nephrolithiasis. Urology. 2011;78:1029–1033.
- Strope SA, Wolf JS Jr, Hollenbeck BK. Changes in gender distribution of urinary stone disease. Urology. 2010;75:543–546.
- Scales CD Jr, Curtis LH, Norris RD, . Changing gender prevalence of stone disease. J Urol. 2007;177:979–982.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328:833–838.
- Kaul P, Sidhu H, Sharma SK, Nath R. Calculogenic potential of galactose and fructose in relation to urinary excretion of lithogenic substances in vitamin B6 deficient and control rats. J Am Coll Nutr. 1996;15:295–302.
- Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425.
- Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905–915.
- Asplin JR. Obesity and urolithiasis. Adv Chronic Kidney Dis. 2009;16:11–20.
- Cignarelli M, Lamacchia O. Obesity and kidney disease. Nutr Metab Cardiovasc Dis. 2007;17:757–762.
- Alberti KG, Eckel RH, Grundy SM, . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645.
- West B, Luke A, Durazo-Arvizu RA, Cao G, Shoham D, Kramer H. Metabolic syndrome and self-reported history of kidney stones: The National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008; 51:741–747.
- Jeong IG, Kang T, Bang JK, . Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis. 2011;58:383–388.
- Okamoto M, Kohjimoto Y, Iba A, Saji F, Hara I, Shigematsu T. Calcium oxalate crystal deposition in metabolic syndrome model rat kidneys. Int J Urol. 2010;17:996–1003.
- Afsar B, Yilmaz M, Eyileten T. Do patients with uric acid stones exhibit abnormal circadian blood pressure – a hypothesis. Nephrol Dial Transplant. 2009;24:2004–2005.
- Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888.
- Abate N, Chandalia M, Cabo-Chan AV Jr, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392.
- Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979.
- Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235.
- Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol. 2010;183:2244–2248.
- Daudon M, Lacour B, Jungers P. High prevalence of uric acid calculi in diabetic stone formers. Nephrol Dial Transplant. 2005;20:468–469.
- Daudon M, Traxer O, Conort P, Lacour B, Jungers JP.Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17:2026–2033.
- Vollans SR, Sehjal R, Forster JA, Rogawski KM. Emphysematous pyelonephritis in type II diabetes: a case report of an undiagnosed ureteric colic. Cases J. 2008;1:192.
- Chan AC, Rohan MJ, Hamid A, Azam A. Emphysematous pyelonephritis in a diabetic patient with pelvic-ureteric stone. Med J Malaysia. 2007;62:166–167.
- Chung SD, Liao CH, Sun HD, Wen WC. Emphysematous pyelonephritis with acute renal failure. Urology. 2008;72:521–522.
- Serrano PE, Khuder SA, Fath JJ. Obesity as a risk factor for nosocomial infections in trauma patients. J Am Coll Surg. 2010;211:61–67.
- Bochicchio GV, Joshi M, Shih D, Bochicchio K, Tracy K, Scalea TM. Reclassification of urinary tract infections in critically ill trauma patients: a time-dependent analysis. Surg Infect (Larchmt). 2003;4:379–385.
- Semins MJ, Shore AD, Makary MA, Weiner J, Matlaga BR. The impact of obesity on urinary tract infection risk. Urology. 2012;79:266–269.
- Haidinger G, Temml C, Schatzl G, . Risk factors for lower urinary tract symptoms in elderly men. For the Prostate Study Group of the Austrian Society of Urology. Eur Urol. 2000;37:413–420.
- Aboumarzouk OM, Somani B, Monga M. Safety and efficacy of ureteroscopic lithotripsy for stone disease in obese patients: a systematic review of the literature. BJU Int. 2012. doi: 10.1111/j.1464-410X.2012.11086.x. [Epub ahead of print].
- El-Assmy AM, Shokeir AA, El-Nahas AR, . Outcome of percutaneous nephrolithotomy: effect of body mass index. Eur Urol. 2007;52:199–204.
- Duvdevani M, Nott L, Ray AA, Ko R, Denstedt JD, Razvi H. Percutaneous nephrolithotripsy in patients with diabetes mellitus. J Endourol. 2009;23:21–26.
- Krambeck AE, Gettman MT, Rohlinger AL, Lohse CM, Patterson DE, Segura JW. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol. 2006;175:1742–1747.
- de CM, Krambeck AE, Rule AD, . Shock wave lithotripsy and diabetes mellitus: a population-based cohort study. Urology. 2012;79:298–302.
- Sato Y, Tanda H, Kato S, . Shock wave lithotripsy for renal stones is not associated with hypertension and diabetes mellitus. Urology. 2008;71:586–591.
