Abstract
Background: Gastrointestinal complications are frequent in patients with renal disease and are responsible for substantial morbidity and mortality among these patients in developing countries. Many times, these patients are subjected to endoscopic evaluation and mucosal biopsies are taken for definitive diagnosis. This study explores the various upper and lower nonneoplastic gastrointestinal complications in patients with chronic kidney disease (CKD) and the role of endoscopic mucosal biopsies in the management of these patients. Methods: Sixty-three patients with CKD, who were referred to endoscopic evaluation and biopsy due to significant gastrointestinal symptoms between January 2007 and December 2011 form the study group. Patients were divided into two groups: group 1 and group 2. Group 1 consisted of patients with CKD stages 1–5 and group 2 consisted of renal allograft recipients. All biopsies were reviewed by an experienced pathologist. Clinical data were collected from patient’s medical records. Results: There were 38 patients in group 1 and 25 patients in group 2. Twenty-nine out of 38 patients in group 1 presented with upper gastrointestinal (GI) symptoms and underwent esophagogastroduodenoscopy (OGD) evaluation, which showed erosive gastritis as the most common biopsy finding followed by ulcerative esophagitis and duodenitis. These patients were also found to be susceptible to develop ischemic colitis due to hypotensive episodes during dialysis, which are likely to occur during the initial stages of dialysis. The most frequent symptom in group 2 was chronic diarrhea (13 out of 25 patients) for which a colonoscopic examination was done which revealed various infection and drug-related colitis, mycophenolate mofetil (MMF) being a major culprit. Conclusion: CKD patients with high urea level are prone to develop upper GI symptoms and mostly show erosive gastritis, ulcerative esophagitis, and duodenitis on biopsy. On the contrary, renal allograft recipients mostly develop opportunistic infections and drug-related toxicity in the colon. MMF-related GI toxicity is an underrecognized entity and further prospective studies are required for its better understanding.
INTRODUCTION
Gastrointestinal (GI) complications are well known to occur in patients with renal disease. Chronic kidney disease (CKD) patients as well as renal allograft recipients form a significant proportion of patients attending endoscopic clinic. This study looks into the various upper and lower nonneoplastic GI complications in these patients who underwent endoscopic mucosal biopsy evaluation.
MATERIALS AND METHODS
Patients who were referred from nephrology unit for endoscopic evaluation, between January 2007 and December 2011, due to significant gastrointestinal symptoms, were selected from the endoscopy register. Inclusion criteria were those patients with irreversible reduction in kidney function, renal allograft recipients, and in whom an endoscopic biopsy was performed. Seventy-six patients fulfilled these criteria during this period. Patients were divided into two groups: group 1 and group 2. Group 1 consisted of patients with CKD stages 1–5 and group 2 consisted of renal allograft recipients who were on immunosuppressant regime. Clinical data were collected from patient’s medical records with particular emphasis on presenting symptoms, serum creatinine level at the time of presentation, comorbid conditions, recent history of any gastric irritant drugs such as antiinflammatory drugs, time interval between commencement of immunosuppressants and time of presentation (in group 2), endoscopic findings, treatment, and outcome. Ten patients were excluded due to difficulty in retrieving patient’s medical records. Additionally, three patients with biopsy diagnosis of neoplastic lesions were also excluded. Mucosal biopsies from the remaining 63 patients were reviewed by an experienced pathologist. All biopsies were examined at two levels using hematoxylin and eosin stain. Additionally, Giemsa stain was used in all gastric biopsies for the identification of Helicobacter pylori.
RESULTS
Group 1
There were 38 patients in group 1 (24 males and 14 females) aged between 21 and 79 years and 23 patients were on maintenance hemodialysis. Average serum creatinine level in nondialyzed patients at the time of biopsy was 3 mg/dL. Five patients were seropositive for hepatitis C infection. Other than the two patients who had adult polycystic kidney disease and one patient with lupus nephritis, the cause for CKD was not documented. Type 2 diabetes mellitus and hypertension were strong comorbid conditions in this group, present in 27 and 34 patients, respectively; others included ischemic heart disease in eight patients, tuberculosis in four patients (one with pulmonary and four with lymph node involvement), and hypothyroidism in three patients. Twenty-nine patients in this group presented with upper GI symptoms, which included dyspepsia, vomiting, dysphagia, chronic anemia, and melena, and underwent esophagogastroduodenoscopy (OGD) evaluation followed by biopsy. Lower GI symptoms were fewer in this group and consisted of diarrhea and bleeding per rectum, for which eight patients underwent colonoscopic biopsies. One patient underwent both OGD and colonoscopic biopsy.
Group 2
There were 25 patients in group 2 (19 males and 6 females) aged between 24 and 62 years. Three patients were seropositive for hepatitis C infection. Nineteen patients were hypertensive and 11patients had posttransplant diabetes. One patient had associated hypothyroidism and another patient had pancreatitis. All patients were on triple immunosuppressant regime, which included prednisolone, tacrolimus, and mycophenolate mofetil (MMF) at the time of biopsy. The time interval between the commencement of immunosuppressants and time of biopsy varied over a wide period from 1 month to 9 years. Five patients had graft dysfunction consistent with chronic allograft nephropathy. In others, the average serum creatinine level at the time of biopsy was 1.76 mg/dL. Chronic diarrhea was the most common indication for endoscopic evaluation in this group and other documented symptoms were abdominal pain, dysphagia, dyspepsia, vomiting, and chronic anemia. Twelve patients had OGD biopsy, 10 patients had colonoscopic biopsy, and 3 patients had both. Biopsy findings in the two groups are presented in .
Table 1. Biopsy findings and diagnosis of groups 1 and 2.
Figure 1. Photomicrograph showing exfoliated esophageal squamous epithelial cells with herpes simplex nuclear inclusions (arrow), hematoxylin eosin stain, 400×.
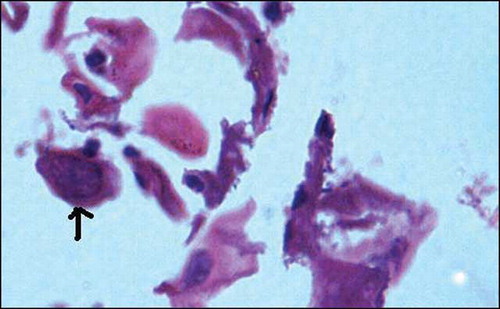
Figure 2. Duodenal mucosal biopsy showing strongyloides larvae within glands (arrow), hematoxylin eosin stain, 200×.
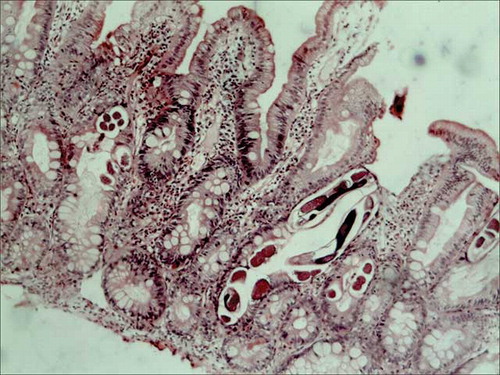
Figure 3. Photomicrograph showing infarcted colonic mucosa in ischemic colitis with adjacent mucosal hemorrhage, hematoxylin eosin stain, 200×.
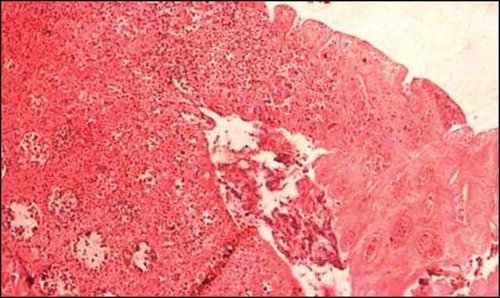
Figure 4. Colonic mucosal biopsy showing CMV inclusions in mucosal capillary endothelium (arrow), hematoxylin eosin stain, 400×.
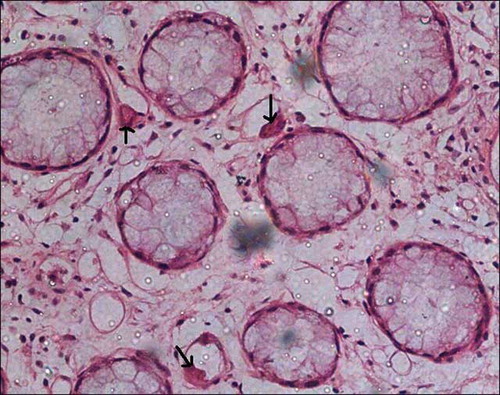
Figure 5. Colonic mucosal biopsy showing marked crypt architectural distortion, branching and clustering of crypts, intervening widely spaced lamina propria shows edema and inflammation, features reminiscent of idiopathic inflammatory bowel disease, hematoxylin eosin stain, 200×.
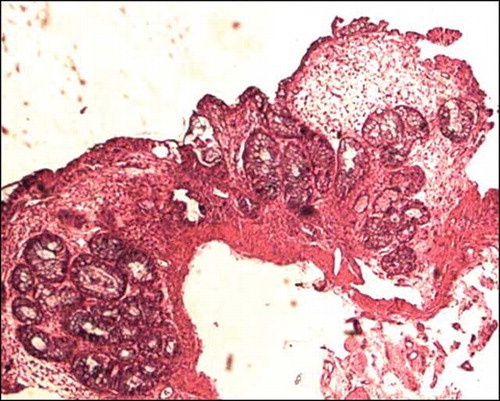
Figure 6. Colonic mucosal biopsy showing significant reduction in crypts, consistent with MMF-related toxic changes, hematoxylin eosin stain, 200×.
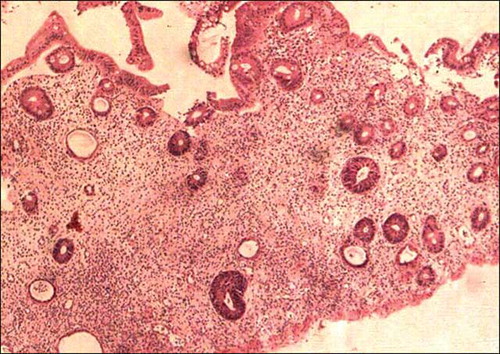
Follow-up data were available for 43 patients; and the shortest follow-up period was 1 month. Ten patients in group 1 and 7 in group 2 had expired. Two patients (one patient in group 1 and one patient in group 2) who expired had acute hemorrhagic gastritis in the biopsy in addition to two patients (group 1) with ischemic colitis and one patient (group 1) with pseudomembranous colitis. There were two patients in group 2 who developed cytomegalovirus (CMV) colitis (), one recovered following antiviral treatment along with the reduction in immunosuppressive medication. The other patient had coexisting graft dysfunction and died of multiorgan failure. Other deaths were due to non-GI complications.
Five patients in group 2 showed very unusual and remarkably similar colonic morphological changes in the biopsy. There was a significant crypt architectural distortion with branching, shortening, and crypt clustering and significant crypt loss with mild to moderate lamina propria inflammation ( and ). These changes were similar to that described with MMF toxicity. One patient (case 2) responded to some extent with reduction in the dosage of MMF, but subsequently developed severe leucopenia necessitating drug withdrawal. MMF toxicity was not suspected in other four patients at the reporting time of biopsy and was given symptomatic treatment and antibiotics with a presumptive diagnosis of longstanding infective colitis. Clinical presentation, endoscopic and biopsy findings, and follow-up of those five patients are tabulated in .
Table 2. MMF-related colitis.
DISCUSSION
CKD patients receiving hemodialysis treatment consist of more than 1.1 million people in the world, and the size of this population is expanding at a rate of 7% per year according to the progress in medical and dialysis machine technique.Citation1 GI complications are known to commonly occur in patients with renal failure. Uremia and dialysis have been found to increase the risk of lesions in the GI tract. In this study, which spans over a 5 year period, 30 out of 38 patients with CKD had significant findings on upper endoscopy and were biopsied. Predominant biopsy findings included erosive gastritis (13), ulcerative esophagitis (7), esophageal candidiasis (8), and duodenitis/duodenal ulcerations (7). None of these patients were documented to be on any gastric irritant drugs. The low incidence of esophageal candidiasis in this study is probably due to the fact that they are not routinely biopsied in our hospital. On the contrary, these GI complications were lower in renal transplant recipients who had relatively stable renal functions as assessed by serum creatinine levels. This finding is in concurrence with that described in the literature. In a large study of 474 subjects (71 chronic renal failure, 73 hemodialysis, 25 renal transplant recipients, and 305 controls) from Baqyiatallah Hospital, Tehran,Citation2 Khedmat et al. found that the erosive gastritis is more common in the uremic subjects [chronic renal failure (CRF): 23.9%; hemodialysis (HD): 30.1%] than those of the other subjects [renal transplant (RT) recipients: 16%; controls: 8.2%]; p < 0.001. Duodenal ulcer in the uremic patients (CRF: 16.1%; HD: 13.7%) was also more common than that in the RT recipients (8%) and controls (6.5%); p = 0.038.
The etiologies of GI complications in CKD are likely to be multifactorial. Urea causing increased back-diffusion of hydrogen ions across the mucosal barrier and elevated gastrin levels partly due to the decreased renal clearance of gastrinCitation3–5 and partly due to the enhanced gastrin synthesis,Citation6 which in turn act as an important stimulus for acid secretion are some of the factors linked to gastric mucosal injuries in renal failure. Since gastrin decreases pyloric sphincter tone, high gastrin levels may lead to biliary reflux, thus worsening mucosal injury.Citation7
H. pylori is a major cause of gastritis in the general population. The increase in the availability of urea, a substrate for H. pylori metabolism, might be expected to increase the prevalence of H. pylori infection in the CKD population. However, recent consensus shows that the prevalence of H. pylori infection in chronic renal failure patients receiving peritoneal or hemodialysis to be equal or lower compared with the subjects with normal renal function in various different geographic populations irrespective of the presence/absence of gastric symptoms.Citation8–11 In our study, only 2 out of 30 patients with CKD (one on hemodialysis), who had OGD biopsy, showed H. pylori infection. The prevalence of infection decreases as dialysis periods progressed, in particular within the first 4 years after the start of treatment, suggesting that hemodialysis treatment, but not uremia, plays a role in the lower prevalence of H. pylori infection.Citation12 Kidney transplant patients with sufficient renal function also have a lower H. pylori infection rate and none of the transplant patients in our group showed H. pylori infection. This may be explained by a situation in which most patients received hemodialysis and/or peritoneal dialysis anytime before kidney transplantation.
There were five cases of ischemic colitis in CKD patients in our study, four patients were on maintenance hemodialysis; one patient died during the same hospital stay due to multiorgan failure. Renal failure patients on hemo or peritoneal dialysis are prone to develop this complication due to repeated bouts of hypotension during dialysis. It is interesting to note that all four patients developed this complication within 1 week of commencement of dialysis. This is understandable due to the fact that these patients stabilize after a few weeks with less frequent hypotensive episodes. The fifth patient, who also died in the immediate postbiopsy period, had a history of radiation for prostatic cancer and ischemia was regarded as secondary to the postradiation stricture. A predilection for right colon ischemia is reported in dialysis patients.Citation13 All five biopsies in our group were sigmoidoscopic biopsies from the left colon.
GI complications in renal allograft recipients are mostly caused by infections and drug toxicity. Opportunistic infections such as CMV are well known to occur in these patients, which can have a detrimental effect on graft/patient survival. MMF-related colitis is emerging as a distinct entity that can display histological features remarkably similar to idiopathic inflammatory bowel disease.Citation14–17 In a large study examining the spectrum of histologic changes associated with MMF therapy in 32 solid organ transplant patients on MMF,Citation15 nine (9/32 patients, 28%) patients showed an inflammatory bowel disease-like pattern with architectural distortion (9/9 patients, 100%), crypt abscesses (4/9 patients, 44%), Paneth cell metaplasia (7/9 patients, 78%), and crypt loss (2/9 patients, 22%). Five allograft recipients in our study, who were on triple immunosuppressants, had similar biopsy findings. All these patients presented with diarrhea of varying duration and colonoscopy showed moderate colitis and multiple aphthae throughout the colon (). Biopsy findings included striking crypt distortion with shortened and branching crypts and significant crypt loss with mild to moderate active inflammation ( and ).
MMF, which was introduced in the mid-1990s in solid-organ transplantation immune-suppression regimen, has led to a significant decrease in the incidence of acute allograft rejectionCitation18 but side effects include gastrointestinal toxicity in the form of nausea, vomiting, and diarrhea. Physicians tend to reduce the dose of MMF or switch their patients to an entero-coated formula to overcome these side effects.Citation19 Because GI side effects are well linked to MMF, colonoscopy is not utilized in most of the cases to investigate the diarrhea and, therefore, the morphological changes still remain unrecognized.
Hardinger et al. in their study looking into the long-term outcome of GI complications in renal transplant patients treated with MMFCitation20 found a reduction in 4-year graft survival from 87.1% to 70.2% (p < 0.0001) with GI complications and MMF discontinuation. In our study, one patient who had associated leucopenia necessitating the withdrawal of MMF developed deterioration in graft functions and is currently on maintenance dialysis, waiting for a second transplant. Another patient who had persistent GI symptoms required readjustment of immunosuppressants and at present has graft dysfunction. Other two patients symptomatically responded to antibiotic treatment and remain stable with normal graft functions and no colonic symptoms, after a period of 1 year and 5 months and 2 years follow-up. However, a follow-up endoscopy/biopsy was not done in any of these patients and therefore the actual status of colonic morphology after treatment is not known. The fifth patient had graft dysfunction with a creatinine level of 5.7 mg/dL at the time of her presentation and was treated with antibiotics, but was lost to follow-up.
The exact pathophysiology of MMF toxicity is not yet clear. It is a prodrug of mycophenolic acid (MPA), an antibiotic substance derived from Penicillium stoloniferous. By inhibiting inosine monophosphate dehydrogenase, an enzyme in the de novo pathway of purine synthesis, MPA prevents the proliferation of T and B lymphocytes and the formation of antibodies from B cells. GI epithelial cells, as well as other cells in the body, are partially dependent on the de novo pathway for growth and replication.Citation21
This study exposes the wide spectrum of GI pathology in patients with kidney disease. There exists a significant difference in GI complications between patients with end-stage renal disease and renal allograft recipients. The former are prone to develop upper GI complications while the latter usually present with chronic diarrhea. Opportunistic infections are well known in allograft recipients; however, morphological effects of drug toxicity remain underrecognized since these patients are not always biopsied. With the emerging reports of rather specific drug-related biopsy changes, endoscopic mucosal biopsies play unquestionable role in the management of these patients.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
REFERENCES
- Lysaght MJ. Maintenance dialysis population dynamics: current trends and long-term implications. J Am Soc Nephrol. 2002;13(Suppl. l):S37–S40.
- Khedmat H, Ahmadzad-Asl M, Amini M, . Gastro-duodenal lesions and Helicobacter pylori infection in uremic patients and renal transplant recipients. Transplant Proc. 2007;39(4):1003–1007.
- Gold CH, Morley JE, Viljoen M, . Gastric acid secretion and serum gastrin levels in patients with chronic renal failure on regular hemodialysis. Nephron. 1980;25:92.
- Muto S, Murayama N, Asano Y, . Hypergastrinemia and achlorhydria in chronic renal failure. Nephron. 1985;40:143.
- Ghonaimy E, Barsoum R, Soliman M, . Serum gastrin in chronic renal failure: morphological and physiological correlation. Nephron. 1985;39:86.
- Paronen I, Ala-Kaila K, Rantala I, . Gastric parietal, chief, and G-cell densities in chronic renal failure. Scand J Gastroenterol. 1991;26:696–700.
- Fisher RS, Lipshutz W, Cohen S. The hormonal regulation of pyloric sphincter function. J Clin Invest. 1973;52:1289.
- Sugimoto M, Yamaoka Y. Review of Helicobacter pylori infection and chronic renal failure. Ther Apher Dial. 2011;15(1):1–9.
- Ozgur O, Boyacioglu S, Ozdogan M, . Helicobacter pylori infection in hemodialysis patients and renal transplant recipients. Nephrol Dial Transplant. 1997;12:289–291.
- Davenport A, Shallcross TM, Crabtree JE, . Prevalence of Helicobacter pylori in patients with end-stage renal failure and renal transplant recipients. Nephron. 1991;59:597–601.
- Sugimoto M, Sakai K, Kita M, Imanishi J, Yamaoka Y. Prevalence of Helicobacter pylori infection in long-term hemodialysis patients. Kidney Int. 2009;75:96–103.
- Chong VH. Impact of duration of hemodialysis on gastrointestinal symptoms in patients with end stage renal failure. J Gastrointestin Liver Dis. 2010;19(4):462–463.
- Flobert C, Cellier C, Berger A, . Right colonic involvement is associated with severe forms of ischemic colitis and occurs frequently in patients with chronic renal failure requiring hemodialysis. Am J Gastroenterol. 2000;95(1): 195–198.
- Dalle IJ, Maes BD, Geboes KP, . Crohn’s-like changes in the colon due to mycophenolate? Colorectal Dis. 2005;7:27–34.
- Selbst MK, Ahrens WA, Robert ME, Friedman A, Proctor DD, Jain D. Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Mod Pathol. 2009;22:737–743.
- Papadimitriou JC, Cangro CB, Lustberg A, . Histologic features of mycophenolate mofetil-related colitis: a graft versus host disease-like pattern. Int J Surg Pathol. 2003;11:295–302.
- Kim HC, Park SB. Mycophenolate mofetil-induced ischemic colitis. Transplant Proc. 2000;32:1896–1897.
- European Mycophenolate Mofetil Cooperative Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. Lancet. 1995;345:1321–1325.
- Calvo N, Sanchez-Fructuoso AI, Conesa J, . Renal transplant patients with gastrointestinal intolerability to mycophenolate mofetil: conversion to enteric-coated mycophenolate sodium. Transplant Proc. 2006;38:2396–2397.
- Hardinger KL, Brennan DC, Lowell J, Schnitzler MA. Long-term outcome of gastrointestinal complications in renal transplant patients treated with mycophenolate mofetil. Transpl Int. 2004;17(10):609–616.
- Allison AC, Eugui EM. The design and development of an immunosuppressive drug, mycophenolate mofetil. Springer Semin Immunopathol. 1993;14:353–380.
