Abstract
Background: Peripheral arterial disease (PAD) frequently occurs in patients on hemodialysis (HD); however, little is known about the effectiveness of drugs. We compare the effects of sarpogrelate and cilostazol in HD patients with PAD. Methods: We conducted a prospective, randomized, open-label, and multicenter trial for 24 weeks in HD patients with PAD. Thirty-five patients were divided into two groups: sarpogrelate (n = 17) and cilostazol (n = 18). We analyzed changes in skin perfusion pressure (SPP), levels of oxidative stress biomarkers, and adverse events. Results: At 24 weeks, SPP was increased in both groups (sarpogrelate, 43 ± 17 to 55 ± 15 mmHg; cilostazol, 49 ± 21 to 66 ± 29 mmHg; p < 0.05), and no difference was observed between the groups. Plasma pentosidine levels decreased in both groups (sarpogrelate, 0.65 ± 0.24 to 0.48 ± 0.12 mg/mL; cilostazol, 0.58 ± 0.22 to 0.47 ± 0.17 mg/mL; p < 0.05), and there were no differences between the groups. Serum malondialdehyde-modified low-density lipoprotein (MDA-LDL) levels significantly increased only in cilostazol group (p < 0.05). There were no clinically significant safety concerns linked to the both drugs. Although blood pressure did not differ in both groups, heart rate increased only in cilostazol group from 77 ± 13 to 83 ± 16 beats per minute (p < 0.05). Conclusion: Sarpogrelate improves SPP in HD patients with PAD without increasing heart rate and serum MDA-LDL levels. We demonstrated that sarpogrelate is an effective and safe drug for the treatment of HD patients with PAD.
INTRODUCTION
Peripheral arterial disease (PAD), which is commonly observed in patients on hemodialysis (HD),Citation1 has a significant impact on mortality. Following the major amputation of a lower limb due to PAD, the 1-year survival rate falls to nearly 50%.Citation2 Another study also demonstrates that the relative risk of mortality among patients who underwent a limb amputation is 1.54 (95% CI = 1.41–1.68; p < 0.001) compared with patients who had not undergone amputation.Citation3 Therefore, it is important to diagnose and begin treating PAD before patients develop symptoms of critical limb ischemia. PAD in HD patients is characterized by distal lesions that are located below the knee or pedal arch, diffuse lesions, and severe vascular calcification,Citation4,5 so that ankle–brachial pressure index (ABI) has limitations for estimating peripheral ischemia.Citation1,5
Measuring skin perfusion pressure (SPP) is the standard method for estimating the sufficiency of microcirculation in ischemic skin. Wounds in which the SPP is below 30 mmHg do not properly heal.Citation6 Moreover, SPP has been used widely to detect early-phase PAD. We compared SPP measurements and multidetector-row computed tomography (MDCT) findings in HD patients and compared the sensitivity and specificity of each method.Citation1 A total of 41.4% of HD patients had an SPP of less than 50 mmHg, with a sensitivity of 84.9% and a specificity of 76.9%. Therefore, SPP is an important objective method to detect PAD as early as possible instead of ABI.
The Inter-Society Consensus for the Management of PAD [Trans-Atlantic Inter-Society Consensus II (TASC II)] recommends the use of antiplatelet agents, such as cilostazol and serotonin type 2 inhibitors, naftidrofuryl, as drug treatment options because these drugs show evidence-based clinical utility in intermittent claudicationCitation7; however, little is known regarding the efficacy of these drugs in HD patients. Sarpogrelate hydrochloride, a selective 5-HT2A antagonist,Citation8 is an antiplatelet agent that improves ischemic symptoms, such as intermittent claudication.Citation9,10 The effect of sarpogrelate in HD patients with PAD has not been elucidated. This study aimed to compare the efficacy and tolerability of the 24-week administration of sarpogrelate and cilostazol in HD patients with PAD. In order to evaluate the efficacy of drug intervention, we measured SPP and levels of oxidative stress biomarkers.
METHODS
Subjects and Methods
Study population
Subjects were considered eligible for the study when they fulfilled the following inclusion criteria: (1) HD patients with PAD who showed at least one symptom (cool sensation in the limbs or intermittent claudication, that is, Fontaine classifications I or II); (2) HD patients with PAD who fulfilled at least one of the following four criteria: (a) levels of SPP of the instep or sole below 50 mmHg, (b) ABI below 1.0, (c) peripheral artery stenosis luminal diameter less than 50% according to MDCT angiography or percutaneous transluminal angiography, and (d) peripheral artery stenosis detected by pulse-wave Doppler ultrasonography with wave pattern types 3 or 4; and (3) HD patients who could stop the administration of antiplatelet agents except aspirin. Exclusion criteria included patients diagnosed with HD within the past 3 months and with clinically apparent worsening ischemic leg symptoms, chronic heart failure, bleeding disorders, hepatic disorders, malignancy, pregnancy, and cerebrovascular disease. Patients were also excluded from the study if they had a history of hypersensitivity to drugs or if they were judged otherwise inappropriate for the study. Patients who could not be evaluated with SPP due to involuntary leg movement were also excluded.
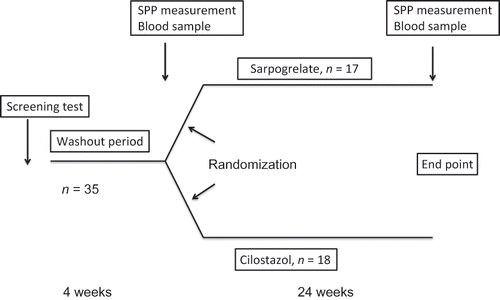
During the entry period from August 2009 to October 2009, a total of 35 HD patients with PAD from two hospitals (Shonan Kamakura General Hospital and Shonan Atsugi Hospital) and one outpatient HD clinic (Shonan Kasama Clinic) fulfilled the study criteria. Written informed consent for participation was obtained from all subjects. We conducted a prospective, randomized, open-label, and multicenter trial for 24 weeks in HD patients with PAD. These 35 PAD patients with HD were randomly divided into two groups, a sarpogrelate group (300 mg/day, n = 17) and a cilostazol group (200 mg/day, n = 18).
Protocol
Subjects who had been previously treated with an antiplatelet agent other than aspirin (e.g., cilostazol, sarpogrelate, beraprost sodium, ticlopidine, or clopidogrel) stopped administration of these drugs for 4 weeks. Only aspirin (100 mg/day) was continued if the subjects had previously been treated with aspirin. Four weeks after the washout period, the subjects were treated with sarpogrelate or cilostazol for 24 weeks ().
Figure 1. Study design. We enrolled 35 patients in this study. These 35 patients with hemodialysis were randomly divided into two groups, a sarpogrelate group (n = 17) and a cilostazol group (n = 18). Antiplatelet agents other than aspirin were stopped for 4 weeks as a washout period. Only aspirin was continued if the patients had previously been treated with aspirin. Four weeks after the washout period, the patients were treated with sarpogrelate or cilostazol for 24 weeks. All 35 patients finished the study.
Subjects were evaluated with SPP at entry into the study and at 24 weeks. Routine examinations were performed at study entry and at 24 weeks. Additionally, serum levels of high-sensitivity C-reactive protein (hsCRP) and malondialdehyde-modified low-density lipoprotein (MDA-LDL) and plasma levels of fibrinogen and pentosidine were measured at study entry and at 24 weeks. Serum MDA-LDL levels were measured with an MDA-LDL ELISA kit (Sekisui Medical Co., Ltd., Tokyo, Japan), and plasma pentosidine levels were measured with a pentosidine ELISA kit (Fushimi Seiyakujo, Marugame, Japan).
The primary end point of our study was an examination of whether SPP values improved in both groups at 24 weeks. Secondary end points included levels of oxidative stress biomarkers at 24 weeks and adverse events during the study period. The study protocol was approved by the local ethical committee of each hospital.
Skin perfusion pressure
SPP was measured using a laser Doppler (PAD 3000, Kaneka Corporation, Tokyo, Japan). PAD 3000 automatically measures SPP using a laser Doppler transmitter and detector set with a pressure cuff. Two points were evaluated in each subject: (a) a point between the first and second metatarsal bones in the instep and (b) a front middle point in the sole. After first inflating the cuff pressure in order to stop skin perfusion, the cuff pressure was deflated, and the point of cuff pressure when skin perfusion restarted was measured. SPP was expressed as the pressure at which skin perfusion restarted. SPP can show capillary perfusion 1.0 mm beneath the skin surface. Each subject was measured for SPP at four points, which included the insteps and soles of both legs. In this study, we used the lowest SPP data of the four points at study entry and compared these values to the same measurement points at 24 weeks for each subject.
Statistical Analyses
All data were expressed as mean ± SD for data showing a normal distribution. When hsCRP levels did not show normal distributions, logarithmic-converted values were used. We also calculated the percent change rate [(value at 24 weeks − value at study entry)/(value at study entry × 100)]. A Mann–Whitney U-test (intragroup comparison) or a Wilcoxon signed-ranked test (comparison between study entry and 24 weeks) were used. Categorical variables were compared using a chi-square test and a Fisher’s test. Stat View 5.0 software for Windows (SAS Institute, Inc., Cary, NC, USA) was used for data analyses on a personal computer. p-Values less than 0.05 were considered significant.
RESULTS
Clinical Characteristics
shows the baseline patient characteristics at study entry. Age, sex, and HD duration did not differ between the sarpogrelate and cilostazol groups. The prevalence of diabetes mellitus and hypertension was not significantly different between the two groups. Additionally, the Fontaine severity classification did not differ between the two groups. ABI values were similar in both groups.
Table 1. Basic patient characteristics.
Primary End Point: Changes in SPP
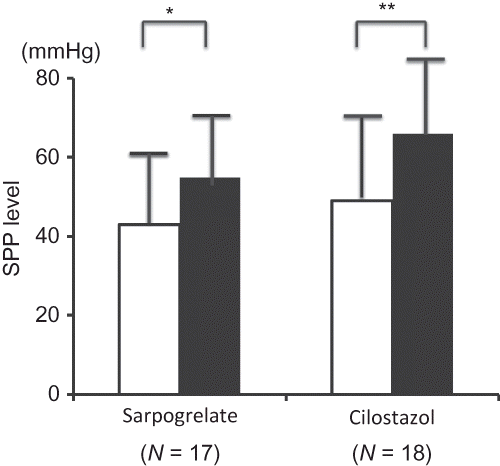
Basal levels of SPP were not different between the two groups. SPP levels significantly increased in both groups at 24 weeks from their basal levels in each group (). SPP levels increased from 43 ± 17 mmHg (pretreatment) to 55 ± 15 mmHg (24 weeks) in the sarpogrelate group (p < 0.05) and from 49 ± 21 mmHg (pretreatment) to 66 ± 29 mmHg (24 weeks) in the cilostazol group (p < 0.01). There were no differences between the two groups at 24 weeks. The percent change rates of SPP were 51 ± 83% in the sarpogrelate group and 41 ± 50% in the cilostazol group. There was no difference between the groups.
Figure 2. Changes in SPP. Data are expressed as mean ± SD.
Notes: Open squares indicate SPP levels at pretreatment and filled squares indicate mean SPP levels at 24 weeks. *p < 0.05, **p < 0.01 versus pretreatment values.
SPP levels significantly increased at 24 weeks from pretreatment levels in both groups.
Secondary End Points
Changes in levels of serum hsCRP, plasma fibrinogen, plasma pentosidine, and serum MDA-LDL
shows the changes in inflammation, rheology of vessels, and oxidative stress marker levels. Serum hsCRP levels and plasma fibrinogen levels significantly increased from their basal levels in the cilostazol group at 24 weeks; however, the changes in these values were not statistically significant in the sarpogrelate group.
Table 2. Changes in serum and plasma parameters.
Plasma pentosidine levels significantly decreased in both groups (0.65 ± 0.24 to 0.48 ± 0.12 μg/mL and 0.58 ± 0.22 to 0.47 ± 0.17 μg/mL in the sarpogrelate and cilostazol groups, respectively; p < 0.01), and there was no difference between the groups. Serum MDA-LDL levels did not significantly change in the sarpogrelate group, while the levels significantly increased from 60.5 ± 25.5 (pretreatment) to 87.0 ± 56.5 U/L (24 weeks) in the cilostazol group (p < 0.05).
Adverse Events
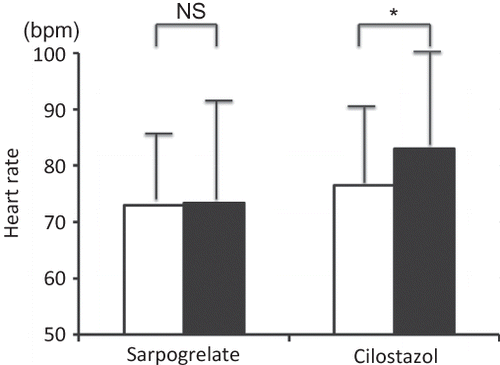
Two of the 18 patients treated with cilostazol complained of headaches at the initiation of the medication administration; therefore, we changed the dose of cilostazol to 50 mg/day and gradually increased the dose to 200 mg/day over 2 weeks. These patients continued taking the medication. There were no major adverse events including bleeding events in either group. Although blood pressure did not differ in both groups, heart rate significantly increased in the cilostazol group from 77 ± 13 beats per minute (bpm) to 83 ± 16 bpm (p = 0.04), but this did not occur in the sarpogrelate group [from 73 ± 14 bpm to 73 ± 17 bpm (p = 0.49)] ().
Figure 3. Changes in heart rate. Data are expressed as mean ± SD. Heart rate in the sarpogrelate group and in the cilostazol group.
Notes: Open squares indicate heart rates at pretreatment and filled squares indicate heart rates at 24 weeks.
*p < 0.05 versus pretreatment value; NS, not significant.
No significant differences were observed between the two groups at pretreatment and at 24 weeks. In the cilostazol group, heart rate significantly increased at 24 weeks.
DISCUSSION
This study demonstrated that (1) sarpogrelate and cilostazol improved the SPP in HD patients with PAD, (2) sarpogrelate and cilostazol improved plasma pentosidine levels, and (3) sarpogrelate did not increase heart rate and serum MDA-LDL levels, whereas cilostazol increased both of these.
The prevalence of PAD in HD patients has been increasing, and PAD significantly affects the prognosis of HD patients.Citation11 PAD is associated with the risk of cardiovascular mortality, morbidity, and hospitalization. Moreover, it is difficult to detect walking impairment in HD patients because they often have arthralgia due to amyloidosis and walking distances are too short to reveal intermittent claudication. PAD in HD patients is characterized by distal lesions located below the knee or pedal arch, diffuse, and long lesions, and severe vascular calcification.Citation4,5 These characteristics in HD patients also increase the difficulty of performing percutaneous transluminal angioplasty.Citation4 Therefore, it is important to diagnose and treat PAD during early phases of this disease.
ABI is widely used as a tool for detecting PAD in the general population. However, these values do not correlate with PAD severity among HD patients. Furthermore, it may be difficult to use ABI to detect isolated obstructions in one or even two of the three branches of the popliteal arteries between the knee and ankle or obstructions in more distal vessels in the foot.Citation12,13 In contrast, SPP is a more sensitive and specific method for evaluating PAD in patients with HD. This method reflects blood perfusion pressure at the arteriolar level in the skin, and it is not affected by arterial calcification.Citation1
Traditionally, SPP has been used to determine the potential for wound repair.Citation6 It is considered difficult to repair wounds in which the SPP is below 30 mmHg. Wound repair may be successful if the SPP level of the lesion is greater than 40 mmHg. Moreover, SPP has been used widely to detect PAD in its early phase.Citation1 Therefore, we focused on changes in SPP levels following the administration of antiplatelet agents. Increasing SPP in ischemic limbs without invasive procedures, such as percutaneous transluminal angioplasty or bypass surgery, may be beneficial for patients with PAD.
Serotonin, which is an endogenous monoamine that is stored in circulating platelets, has a variety of physiological effects. Once platelets are activated at sites of endothelial injury, serotonin is released from activated platelets, and it plays an important role in thrombotic occlusion.Citation14 In PAD and diabetic patients, serotonin concentrations in platelets are lower than in healthy controls, and plasma serotonin concentrations are increased substantially in these patients.Citation15 Additionally, plasma serotonin is significantly elevated in HD patients.Citation16 Serotonin binds various receptors and mediates both vasoconstriction and vasodilatation.Citation17 Platelet activation and the vasoconstrictive effects of serotonin are mediated by 5-HT2A receptors, which are located primarily on platelets and vascular smooth muscle cells.Citation18
Sarpogrelate hydrochloride, a selective 5-HT2A receptor antagonist, is widely used as an antiplatelet agent for the treatment of PAD.Citation9,10 However, there have been no prospective and randomized interventional studies that provide evidence regarding the efficacy of sarpogrelate or other antiplatelet agents for the treatment of HD patients with PAD. This study demonstrated that sarpogrelate was as equally effective as cilostazol in the treatment of PAD in HD patients.
Plasma pentosidine levels significantly decreased following the administration of sarpogrelate and cilostazol. It is well known that patients with HD are exposed to oxidative stress and inflammation, and oxidative stress has been strongly implicated in atherosclerosis. Advanced glycation end products (AGEs) are produced in the presence of oxidative stress. In end-stage renal disease, serum levels of AGEs are markedly elevated.Citation19 Pentosidine, an AGE, is reportedly associated with extensive coronary artery calcification in HD patients.Citation20 In this study, pentosidine levels decreased following the administration of sarpogrelate and cilostazol; therefore, both drugs lower oxidative stress.
Serum MDA-LDL levels are a reliable marker of lipid peroxidation, and they are also used as a biomarker of oxidative stress. Various coronary risk factors, including serum MDA-LDL concentrations, are considered to be potent risk factors for in-stent restenosis in diabetic patients.Citation21 Serum MDA-LDL may act as a growth factor for neointimal tissues inside an implanted stent. In this study, serum MDA-LDL levels did not change at 24 weeks in the sarpogrelate-treated group, whereas a significant increase was observed in the cilostazol-treated group. Sarpogrelate decreases superoxide anion production from macrophages and neutrophilsCitation22 and increases superoxide dismutase activity and nitric oxide release in isolated rat aorta.Citation23 Sarpogrelate may inhibit nitric oxide scavenging by inhibiting superoxide anion production; thus, sarpogrelate improves endothelial function and significantly decreases serum concentrations of IL-6 and hsCRP in PAD patients.Citation24 In this study, serum hsCRP levels and plasma fibrinogen levels significantly increased in the cilostazol group; in contrast, these values did not change in the sarpogrelate group. Serotonin likely plays an important role in vascular inflammation associated with atherosclerosis. Acceleration of the oxidation stress and inflammatory reactions in HD patient was suppressed in the sarpogrelate group. Therefore, platelet activation was suppressed, and plasma fibrinogen levels did not significantly change in the sarpogrelate group.
There were no clinically significant safety concerns linked to the drugs, confirming good tolerability of sarpogrelate and cilostazol. Only two patients treated with cilostazol complained of headaches at the initiation of the medication administration, but the headaches did not continue for long. However, the heart rate in cilostazol-treated patients increased by 6 bpm (p = 0.04) in this study. Cilostazol is a phosphodiesterase-III inhibitor that increases cAMP levels, resulting in an increased heart rate. It was recently demonstrated that, in chronic HD patients, survival rate decreases with an increased pre-HD pulse rate25; thus, tachycardia is a predictor of poor survival in chronic HD patients in Japan. Therefore, it is extremely important to treat HD patients with drugs that do not increase heart rate. Based on this information, sarpogrelate is more appropriate for treating PAD in HD patients compared to cilostazol.
There are some limitations of this study. The sample size is small and the study period is short. Furthermore, the open label nature of the study is also an important limitation. An additional large-scale, double-blind, and randomized study is necessary in order to confirm the efficacy of sarpogrelate in the treatment of HD patients with PAD. However, this is the first report to study the effect of drugs using SPP in HD patients. Furthermore, we mentioned sarpogrelate has shown no adverse effects. However, small sample size and short duration of follow-up has not allowed capturing relevant but less frequent adverse events of sarpogrelate.
In conclusion, the results of this study demonstrated that sarpogrelate is effective for increasing SPP levels without influencing the heart rate in HD patients with PAD. Additionally, sarpogrelate exhibited a good safety profile.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Okamoto K, Oka M, Maesato K, . Peripheral arterial occlusive disease is more prevalent in patients with hemodialysis: comparison with the findings of multidetector-row computed tomography. Am J Kidney Dis. 2006;48:269–276.
- O’Hare AM, Bertenthal D, Shlipak MG, Sen S, Chren MM. Impact of renal insufficiency on mortality in advanced lower extremity peripheral arterial disease. J Am Soc Nephrol. 2005;16:514–519.
- Combe C, Albert JM, Bragg-Gresham JL, . The burden of amputation among hemodialysis patients in the dialysis outcomes and practice patterns study. Am J Kidney Dis. 2009;54:680–692.
- Leskinen Y, Salenius JP, Lehtimaeki T, Huhtala H, Saha H. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am J Kidney Dis. 2002;40:472–479.
- Ohtake T, Oka M, Ikee R, . Impact of lower limbs’ arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J Vasc Surg. 2011;53:676–683.
- Castronuovo JJ, Adera HM, Smiell JM, Price RM. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg. 1997;24:629–637.
- Norgren L, Hiatt WR, Dormandy JA, . Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26:81–157.
- Hara H, Osakabe M, Kitajima A, Tamao Y, Kikumoto R. MCI-9042, a new antiplatelet agent is a selective S2-serotonergic receptor antagonist. J Thromb Hemost. 1991;65:415–420.
- Norgren L, Jawien A, Matyas L, Riegerd H, Arita K. Sarpogrelate, a 5-hT2A receptor antagonist in intermittent claudication. A phase II European study. Vasc Med. 2006;11:75–83.
- Matsuo H, Shigematsu H. Effects of the 5-HT2A antagonist sarpogrelate on walking ability in patients with intermittent claudication as measured using the walking impairment questionnaire. Ann Vasc Dis. 2008;1:102–110.
- Rajagopalan S, Dellegrottaglie S, Furnisss AL, . Peripheral arterial disease in patients with end-stage renal disease: observations from the dialysis outcomes and practice patterns study (DOPPS). Circulation. 2006;114:1914–1922.
- Kondo Y, Muto A, Dardik A, Nishibe M, Nishibe T. Laser Doppler skin perfusion pressure in the diagnosis of limb ischemia in patients with diabetes mellitus and/or hemodialysis. Int Angiol. 2007;26:258–261.
- Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. J Am Med Assoc. 1969;207: 1869–1874.
- Nishihira K, Yamashita A, Tanaka N, . Inhibition of 5-hydroxytryptamine receptor prevents occlusive thrombus formation on neointima of the rabbit femoral artery. J Thromb Hemost. 2006;4:247–255.
- Barradas MA, Gill DS, Fonseca VA, Mikhailidis DP, Dandona P. Intraplatelet serotonin in patients with diabetes mellitus and peripheral vascular disease. Eur J Clin Invest. 1988;4:399–404.
- Cheng CH, Cheng FC, Shu KH, Wu MJ. Abnormal serotonin metabolism in long-term hemodialysis and CAPD patients. Am J Nephrol. 1997;17:541–542.
- Hoyer D, Clarke DE, Fozard JR, . International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–203.
- Nagatomo T, Rashid M, Abul Muntasir H, Komiyama T. Functions of 5-HT2A receptor and its antagonists in the cardiovascular system. Pharmacol Ther. 2004;104:59–81.
- Makita Z, Bucala R, Rayfield EJ, . Reactive glycosylation endproducts in diabetic uremia and treatment of renal failure. Lancet. 1994;343:1519–1522.
- Taki K, Takayama F, Tsuruta Y, Niwa T. Oxidative stress, advanced glycation end product, and coronary artery calcification in hemodialysis patients. Kidney Int. 2006;70:218–224.
- Shigematsu S, Takahashi N, Hara M, Yoshimatsu H, Saikawa T. Increased incidence of coronary in-stent restenosis in type 2 diabetic patients is related to elevated serum malondialdehyde-modified low-density lipoprotein. Circ J. 2007;71:1697–1702.
- Mikawa K, Akamatsu H, Nishina K, et al. The effects of sarpogrelate on superoxide production by human neutrophils. Reg Anesth Pain Med. 2000;25:181–186.
- Sun YM, Su Y, Jin HB, . Sarpogrelate protects against high glucose-induced endothelial dysfunction and oxidative stress. Int J Cardiol. 2011;147:383–387.
- Miyazaki M, Higashi Y, Goto C, . Sarpogrelate hydrochloride, a selective 5-HT2A antagonist, improves vascular function in patients with peripheral arterial disease. J Cardiovasc Pharmacol. 2007;49:221–227.
- Iseki K, Nakai S, Yamagata K, . Tachycardia as a predictor of poor survival in chronic hemodialysis patients. Neprol Dial Transplant. 2011;26:963–969.
