Abstract
Background: Cardiovascular disease is the main cause of mortality after renal transplantation. Soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) and fibroblast growth factor-23 (FGF-23) are two novel molecules that have been associated with atherosclerosis in different populations. In this cross-sectional study, we investigated the associations between sTWEAK, FGF-23, and carotid artery intima-media thickness (CA-IMT) in renal transplant patients. Methods: A total of 117 renal transplant patients were studied. CA-IMT was determined by B-mode Doppler ultrasonography. Serum sTWEAK and FGF-23 were measured by a commercially available enzyme-linked immunosorbent assay (ELISA). Results: Mean age was 39.6 ± 9.6 years and 51% of the patients were male. Mean sTWEAK level was 595 ± 225 pg/mL (158–1140), FGF-23 level was 92 ± 123 RU/mL (9.6–1006), and CA-IMT level was 0.62 ± 0.11 mm (0.40–0.98). sTWEAK level was positively correlated with CA-IMT. There was no association between sTWEAK and FGF-23 levels. FGF-23 was also associated with CA-IMT. In adjusted models using linear regression analysis, only age and serum TWEAK levels were predictors for CA-IMT. Conclusion: There is a positive correlation between CA-IMT and sTWEAK, but not with FGF-23 levels in renal transplant patients.
INTRODUCTION
Renal transplantation (RT) is considered as the most successful renal replacement therapy method in patients with end-stage renal disease. Cardiovascular burden induced by the uremic status declines, but still continues even after a successful RT and limits patient survival.Citation1 Carotid artery intima-media thickness (CA-IMT) measurements have been shown to be a reliable indicator of atherosclerosis and a predictor for cardiovascular diseases in this group of patients.Citation2 Older age, hypertension, dyslipidemia, graft functions, and inflammation are among the most important risk factors for carotid atherosclerosis in these individuals.Citation3–5
Soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) has recently been associated with atherosclerosis, endothelial dysfunction, and mortality in renal patients. Alike in non-renal individuals,Citation6 studies have shown that low sTWEAK levels are related to atherosclerosis, endothelial dysfunction, and prediction of cardiovascular events in non-CKD patients and nondialyzed CKD stages.Citation7,8 Confusingly, high levels have also been shown to relate to atherosclerosis, vascular calcification, and mortality in dialysis individuals,Citation9,10 suggesting a dual or time-differential effect that agrees nevertheless with observations in non-CKD patients.Citation11 Fibroblast growth factor-23 (FGF-23) is an emerging mediator of the vascular calcification process. Similarly to sTWEAK, a dual effect with regard to carotid atherosclerosis in dialysis patients has been proposed.Citation12,13 It has been proposed that TWEAK activates FGF-23 receptorsCitation14 and thus these two molecules might have complementary effects on atherosclerosis. The associates of these two molecules in renal transplant recipients are poorly investigated. In this study, we assessed the plausible role of sTWEAK and FGF-23 in the pathogenesis of atherosclerosis in RT patients.
PATIENTS AND METHODS
In this cross-sectional study, 117 adult RT patients who responded to our invitation to be involved in this study and met the inclusion criteria were studied. Inclusion criteria were to be transplanted at least 6 months prior to the study and not to have an active infection or an acute rejection episode within the last 3 months. Clinical data were obtained from the patients’ charts. All procedures were performed in concordance with the ethical standards of the Declaration of Helsinki. Blood chemistry parameters were assayed by standardized and automated techniques in the same laboratory registered to several external quality control programs. Level of proteinuria was calculated from spot urine protein/creatinine ratio.
Serum concentrations of sTWEAK were measured in duplicates with a commercially available enzyme-linked immunosorbent assay (ELISA) kit (BenderMed Systems, Vienna, Austria). ELISA was performed according to the manufacturer’s protocol. The minimum detectable level of sTWEAK was 10 pg/mL. Intra- and interassay coefficients of variation were 7.4% and 9.2%, respectively. Serum concentrations of FGF-23 were measured using second-generation Human FGF-23 (C-Term) ELISA kit (ImmunTopics, San Clemente, CA, USA).
Carotid Artery Intima-Media Thickness Measurement
Ultrasonographic studies on common carotid arteries were carried out by gray scale, high-resolution color Doppler ultrasound (ATL HDI 5000 scanner Philips, ATL ultrasound, Bothell, WA, USA) equipped with 5–12 MHz linear transducer on the day of blood sampling. All procedures were performed on both sides of two longitudinal images of the each common carotid artery on the morning by the same operator. Average of two CA-IMT values from each side were used to calculate mean CA-IMT. The most distal 1-cm segment (just proximal to the bulbar dilatation) of both common carotid arteries was scanned on longitudinal plane to achieve IMT values. The echogenic inner line (representing the lumen-intima interface) and adjacent hypoechoic layer (media) of the far wall of the artery were measured together to get the IMT value. Values from both sides were averaged to obtain the mean CA-IMT of each donor. Intra-observer coefficient of variation was 2.32%.
Statistical Analyses
All results are expressed as mean ± SD. A p-value of less than 0.05 was considered statistically significant. Comparisons between two groups were assessed by independent t-test analysis. Spearman correlation analysis was used to assess the correlations of CA-IMT and other variables. The conventional risk factors for CA-IMT along with the studied biomarkers were included in the adjusted models using linear regression analyses. All statistical analyses were performed using SPSS, version 15 (SPSS, Inc., Chicago, IL, USA).
RESULTS
A total of 117 adult renal transplant patients were stu died. Mean age was 39.6 ± 9.6 years and 9% were diabetic. Fifty-two percent of the patients were men. Eighty-five (73%) patients received hemodialysis (HD) and 11 (9.5%) patients received peritoneal dialysis prior to their RT. An additional 19 (16.4%) patients had undergone pre-emptive RT. Seventy- three (62.4%) of the transplantations were performed from living donors and 44 (37.6%) were from cadavers. Fifty-four percent of the patients were receiving calcineurin inhibitor (CNI) therapy + steroids + mycophenolate mofetil-mycophenolate sodium and 15% patients were mammalian target of rapamycin inhibitors + steroids + mycophenolate mofetil-mycophenolate sodium therapies for maintenance treatment. As anti-hypertensive medication, 25 patients were on angiotensin converting enzyme inhibitors or receptor blockers, 76 on calcium channel blockers, 28 on β-blockers, and 18 on diuretics. Thirty-seven (31.9%) patients were receiving combined anti-hypertensive therapy. Thirteen (11.1%) patients were on statins and five patients on fibrate derivative drugs. Six patients received phosphorus-binding agents and five calcitriol. In addition, no patients received anti-coagulant therapy. Three patients were hepatitis B and 14 were hepatitis C positive. Mean posttransplantation follow-up period was 76.1 ± 59.1 months (6–350) and mean serum creatinine level was 1.9 ± 1.2 mg/dL. Demographical, clinical, and laboratory data of the study patients are listed in .
Table 1. Laboratory data of the study population.
Soluble Tumor Necrosis Factor-Like Weak Inducer of Apoptosis
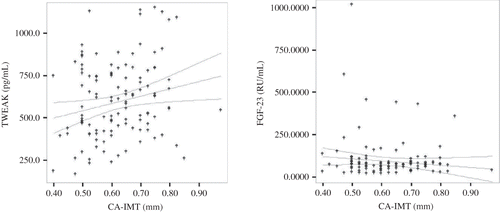
Mean sTWEAK level was 595 ± 225 (158–1140) pg/mL. In univariate analysis (), there was a positive correlation between sTWEAK and glomerular filtration rate (GFR), albumin, calcium, hemoglobin, hematocrit levels, and CA-IMT. sTWEAK level was negatively correlated with history of rejection, serum urea, creatinine, phosphorus, calcium phosphorus product, parathormone (PTH), and proteinuria levels.
Table 2. Correlation analysis of CA-IMT, TWEAK, and FGF-23.
Table 3. Multivariate linear regression analysis of the predictive factors for CA-IMT.
Fibroblast Growth Factor-23
Mean FGF-23 level was 92 ± 123 (9.6–1006) RU/mL. There existed a positive correlation between FGF-23 and PTH, posttransplantation follow-up period, systolic and diastolic blood pressures, serum urea, creatinine, uric acid, phosphorus, calcium phosphorus product, alkaline phosphatase, and proteinuria levels and a negative correlation between FGF-23 and GFR, albumin, cholesterol, hemoglobin, and hematocrit levels ().
Carotid Artery Intima-Media Thickness
Mean CA-IMT was 0.62 ± 0.11 mm (0.40–0.98). CA-IMT was positively correlated with age, history of cardiovascular disease (CVD) and smoking, duration of dialysis, serum albumin, calcium, hemoglobin, hematocrit, and sTWEAK levels and negatively with serum phosphorus and creatinine levels. There was no correlation between CA-IMT and FGF-23. The correlations between CA-IMT and TWEAK, and FGF-23 are presented in .
In the model including conventional risk factors of CA-IMT such as age, gender, diabetes, and smoking histories, duration of dialysis together with follow-up period and sTWEAK level, age, and TWEAK level were found to be the predictors for CA-IMT in analysis using logistic regression method ().
DISCUSSION
In this cross-sectional study, the effects of serum sTWEAK and FGF-23 levels on CA-IMT in 117 adult RT patients were studied. Our results show that there is no association between FGF-23 and CA-IMT, whereas a strong association between sTWEAK levels and CA-IMT was observed.
FGF-23 is a hormone that is secreted from osteoblasts with phosphaturic effects. FGF-23 levels progressively increase as CKD advancesCitation8 and is restored following a successful transplantation.Citation15 Several studies have investigated the relationship between FGF-23 and carotid atherosclerosis in dialysis patients; reporting controversial results. In the study by Ashikaga et al., a negative relation was reported between FGF-23 and CA-IMT in a cohort of 196 HD patients.Citation13 On the contrary, Balci et al. demonstrated a positive relationship between in a similar number of HD patients.Citation12 In these two studies, a positive relationship between FGF-23 levels and serum creatinine, phosphorus, calcium phosphorus product, PTH, and a negative relationship with cholesterol levels was reported. In our study, there was a similar correlation list for FGF-23. Furthermore, a positive relationship between FGF-23 levels and the posttransplantation follow-up period, systolic blood pressure (SBP), diastolic blood pressure (DBP), and proteinuria levels and a negative relationship between FGF-23 levels and hemoglobin and albumin levels was found. Balci et al. suggested that increased FGF-23 levels may lead to an increase in the number of FGF receptors in tunica media of the arteries and cause neointimal hypertrophy and fibrosis.Citation16 FGF-2 is a key mediator of vascular smooth muscle cell (VSMC) proliferation following arterial injury that results in neointimal growth. Moreover, it has been shown that FGF-2 is responsible for a significant part of VSMC proliferation in the tunica media stimulated by endothelial injury to the rat carotid artery.Citation17 Increased expression of basic FGF and FGFR-1 in VSMCs of unstable plaques in carotid arteries has been reported more recently.Citation18 It was claimed that subsequently it causes intimal hyperplasia and contributes to carotid atherosclerosis.Citation12 On the other hand, Ashikaga et al. proposed that FGF-23 levels cause decreases lipid levels by interacting with different receptors, thus, having a negative effect on carotid atherosclerosis.Citation19
The relationship between FGF-23 and CA-IMT has not yet been studied in RT patients. In our study, we could not find a relationship between CA-IMT and FGF-23. This may be explained by the younger patient population in our study having better kidney functions (mean GFR was 50 ± 25 mL/min) compared with those in the other two studies (GFR < 10 mL/dk).Citation10,11 FGF-23 levels start increasing particularly in patients with GFR lower than 30.Citation17 Also, the low FGF-23 levels in our study compared with the levels observed in dialysis patients may not be sufficient enough to lead to receptor activation and therefore cause intimal hypertrophy. As a result, it should not be surprising that FGF-23 does not affect CA-IMT in a posttransplantation patient group with moderate GFR levels.
sTWEAK is a glycoprotein of the tumor necrosis factor superfamily. It is involved in cellular development, proliferation, migration, osteoclastogenesis, angiogenesis, and apoptosis by binding to its receptor, Fn14.Citation20,21 In mice studies, TWEAK was demonstrated to cause nuclear factor kappa B (NF-κB) activation and thus trigger inflammation by stimulating interleukin-6 (IL-6) and mitogen-activated protein kinase phosphatase-1 (MKP-1) production.Citation22 The relationship between sTWEAK and atherosclerosis is not clear. In the study of Blanco-Colio et al., a negative relationship was observed between sTWEAK levels and CA-IMT in a nonrenal population.Citation6 Yilmaz et al. demonstrated that decrease in sTWEAK levels accompany the increase in kidney failure level and that sTWEAK levels are inversely related to endothelial dysfunction.Citation8 Additionally, Carrero et al. demonstrated that increased sTWEAK levels cause an increase in mortality by stimulating inflammation in dialysis patients.Citation9 These studies proposed that TWEAK may either increase the inflammatory activity or be preventive against extreme inflammation depending upon its interaction with tissue receptors and also its expression and secretion may be decreased in atherosclerotic situations.
A positive relationship was determined between sTWEAK and CA-IMT in our study. When the conventional cardiovascular risk factors (age, gender, history of diabetes, duration of dialysis, posttransplantation follow-up period, and smoking history) and sTWEAK levels were inserted into the model in logistic regression analysis to investigate the risk factors for CA-IMT, age, and sTWEAK levels were found as independent predictors. This relationship is probably due to the effects of sTWEAK via the activation of NF-κB and thereafter stimulating the production of IL-6 and other adhesion molecules. Consequently, this triggers inflammation as demonstrated in previous studies.Citation22,23 Carrero et al. claimed that TWEAK triggered inflammation, increased IL-6 levels showed synergic effect with TWEAK and that together they could increase the inflammation to an extreme level.Citation9 Wiley et al. reported that TWEAK-Fn14 interactions were important for phorbol myristate acetate- and epidermal growth factor-stimulated endothelial cell migration as well as FGF-2-mediated angiogenesis in the mouse cornea.Citation20 Indeed, Jakubowski et al. showed that TWEAK was neither a survival factor or a mitogen nor a chemotactic factor for endothelial cells. They also reported that TWEAK co-treatment potentiated FGF-2 an in vitro angiogenesis assay.Citation24
There are limitations of this study that need to be addressed. First is the cross-sectional nature of the study. Second, the relationship between serum levels of sTWEAK and FGF-23 were investigated, but tissue levels and receptor interactions were not.
To conclude, we report that sTWEAK, but not with FGF-23 levels, associates with carotid atherosclerosis in renal transplant patients. Accumulating data on the role sTWEAK in the pathophysiology of cardiovascular in both renal and nonrenal populations is highly promising and further prospective studies with larger sample size may provide information about the causal link of sTWEAK in CVD.
Declaration of interest: Juan Jesus Carrero acknowledges support from the Loo and Hans Ostermans Foundation and the Swedish research council. The others declare no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Jardine AG, Gaston RS, Fellstrom BC, Holdaas H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet. 2011;15(378):1419–1427.
- Toz H, Duman S, Altunel E, . Intima media thickness as a predictor of atherosclerosis in renal transplantation. Transplant Proc. 2004;36:156–158.
- Sánchez-Escuredo A, Pastor MC, Bayés B, . Inflammation, metalloproteinases, and growth factors in the development of carotid atherosclerosis in renal transplant patients. Transplant Proc. 2010;42:2905–2907.
- Yilmaz MI, Qureshi AR, Carrero JJ, . Predictors of carotid artery intima-media thickness in chronic kidney disease and kidney transplant patients without overt cardiovascular disease. Am J Nephrol. 2010;31:214–221.
- Cofan F, Arias M, Nuñez I, . Impact of carotid atherosclerosis as assessed by B-mode ultrasonography on the evolution of kidney transplantation. Transplant Proc. 2007;39:2236–2238.
- Blanco-Colio LM, Martín-Ventura JL, Muñóz-García B, . Identification of soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) as a possible biomarker of subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:916–922.
- Yilmaz MI, Sonmez A, Ortiz A, . Soluble TWEAK and PTX3 in nondialysis CKD patients: impact on endothelial dysfunction and cardiovascular outcomes. Clin J Am Soc Nephrol. 2011;6:785–792.
- Yilmaz MI, Carrero JJ, Ortiz A, . Soluble TWEAK plasma levels as a novel biomarker of endothelial function in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1716–1723.
- Carrero JJ, Ortiz A, Qureshi AR, . Additive effects of soluble TWEAK and inflammation on mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:110–118.
- Gungor O, Kircelli F, Asci G, . Soluble TWEAK level: is it a marker for cardiovascular disease in long-term hemodialysis patients? J Nephrol. 2012;13. doi:10.5301/jn.5000121
- Jain M, Jakubowski A, Cui L, . A novel role for tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in the development of cardiac dysfunction and failure. Circulation. 2009;119:2058–2068.
- Balci M, Kirkpantur A, Gulbay M, Gurbuz OA. Plasma fibroblast growth factor-23 levels are independently associated with carotid artery atherosclerosis in maintenance hemodialysis patients. Hemodial Int. 2010;14:425–432.
- Ashikaga E, Honda H, Suzuki H, . Impact of fibroblast growth factor 23 on lipids and atherosclerosis in hemodialysis patients. Ther Apher Dial. 2010;14:315–322.
- Wiley SR, Cassiano L, Lofton T, . A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 2001;15:837–846.
- Economidou D, Dovas S, Papagianni A, Pateinakis P, Memmos D. FGF-23 levels before and after renal transplantation. J Transplant. 2009;2009:379082.
- Agrotis A, Kanellakis P, Kostolias G, . Proliferation of neointimal smooth muscle cells after arterial injury. Dependence on interactions between fibroblast growth factor receptor-2 and fibroblast growth factor-9. J Biol Chem. 2004;279:42221–42229.
- Lewis CD, Olson NE, Raines EW, Reidy MA, Jackson CL. Modulation of smooth muscle proliferation in rat carotid artery by platelet-derived mediators and fibroblast growth factor-2. Platelets. 2001;12:352–358.
- Sigala F, Savvari P, Liontos M, . Increased expression of bFGF is associated with carotid atherosclerotic plaques instability engaging the NF-κB pathway. J Cell Mol Med. 2010;14:2273–2280.
- Nakai K, Komaba H, Fukagawa M. New insights into the role of fibroblast growth factor 23 in chronic kidney disease. J Nephrol. 2010;23:619–625.
- Wiley SR, Winkles JA. TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TWEAKR/Fn14 receptor. Cytokine Growth Factor Rev. 2003;14:241–249.
- Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine. 2007;40:1–16.
- Muñoz-García B, Moreno JA, López-Franco O, . Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) enhances vascular and renal damage induced by hyperlipidemic diet in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:2061–2068.
- Sanz AB, Sánchez-Nino MD, Izquierdo MC, . TWEAK, the facilitator of acute kidney injury. Nefrologia. 2008;28:587–592.
- Jakubowski A, Browning B, Lukashev M, . Dual role for TWEAK in angiogenic regulation. J Cell Sci. 2002;115: 267–274.