Abstract
The association between endothelial nitric oxide synthase (eNOS) Glu298Asp gene polymorphism and diabetic nephropathy (DN) risk is still controversial. A meta-analysis was performed to evaluate the association between eNOS Glu298Asp gene polymorphism and DN susceptibility. A predefined literature search and selection of eligible relevant studies were performed to collect data from electronic database. Eight articles were identified for the analysis of association between eNOS Glu298Asp gene polymorphism and DN risk. T allele was associated with DN susceptibility in overall populations, in Asians, and for Caucasians (overall populations, p = 0.005; Asians, p = 0.004; Caucasians, p = 0.002). Furthermore, GG genotype might play a protective role against DN onset for overall populations, Asians, Caucasians, and Africans. However, a link between eNOS Glu298Asp gene polymorphism and DN risk was not found in overall populations, Asians, Caucasians, and Brazil population. In conclusion, T allele might become a significant genetic molecular marker for the onset of DN in overall populations, in Asians, and for Caucasians. However, more studies should be performed in the future.
INTRODUCTION
Diabetic nephropathy (DN) is one of the most common microvascular complications of diabetes and the leading cause of end-stage renal disease.Citation1 Susceptibility to DN has an inherent genetic basis as evidenced by familial aggregation and ethnic-specific prevalence rates.Citation2 Some current investigationsCitation3–5 suggested that gene polymorphism might play a key role in the onset of DN.
Nitric oxide (NO) is a ubiquitous vasodilator and an important regulator of renal sodium excretion.Citation6 Reduced NO generation induces renal injury,Citation7 and impairment of endothelial NO generation is considered the major deterioration factor for progressive renal disease. NO is produced by inducible nitric oxide synthase.Citation8 Glu298Asp is an important gene mutation of endothelial nitric oxide synthase (eNOS), and the eNOS Glu298Asp gene polymorphism includes GG (Glu/Glu), GT (Glu/Asp), and TT (Asp/Asp) genotypes and G (Glu) and T (Asp) alleles. We present an epidemiologic study showing that the eNOS Glu298Asp gene polymorphism has been implicated in the etiology of DN. However, the available evidence reported to date is weak, due to sparseness of data or disagreements among studies. There was rare meta-analysis to explore the association of eNOS Glu298Asp gene polymorphism with DN risk. We performed this meta-analysis to investigate the relation between eNOS Glu298Asp gene polymorphism and DN susceptibility, with the intention to provide a much more reliable finding on the significance of the association.
MATERIALS AND METHODS
Search Strategy
The relevant studies were screened from the search engines of PubMed, Embase, Cochrane Library, and CBM-disc (China Biological Medicine Database) on 1 March 2012. The following terms were used to complete the search: “diabetic nephropathy,” “DN,” “diabetes mellitus nephropathy,” “endothelial nitric oxide synthase,” “eNOS,” and “gene.” We also extended search spectrum to the “related articles” and the bibliographies of all retrieved studies. If multiple publications of the same data from the same study group occurred, we only recruited the later paper for analysis.
Inclusion and Exclusion Criteria
Inclusion criteria
(1) A case–control study; (2) the outcome had to be DN; and (3) there had to be at least two comparison groups (DN group vs. control group).
Exclusion criteria
(1) Review articles, editorials, and case reports; (2) articles that did not provide the detailed genotype data; (3) investigating the association of other genes with DN; (4) investigating the role of eNOS in diseases; and (5) multiple publications of the same data from the same study group.
Data Extraction and Synthesis
The following information was extracted from each study independently by at least two investigators: first author’s surname, year of publication, ethnicity of study population, and the number of cases and controls for eNOS genotype. Frequencies of T allele were calculated for case group and control group from the corresponding genotype distribution. The results were compared and disagreements were resolved by discussion.
Statistical Analysis
Available data were entered into Cochrane Review Manager (RevMan, version 5, Oxford, UK) and analyzed. The pooled statistic was counted using the fixed effects model, but a random effects model was conducted when the p-value of heterogeneity test was less than 0.1.Citation9 The results were expressed with odds ratios (ORs) for dichotomous data, and 95% confidence intervals (CIs) were also calculated. A p-value of <0.05 was required for the overall OR to be deemed statistically significant.Citation10,11 I2 was used to test the heterogeneity between the included studies. We classified the investigations into studies for Caucasians, Asians, Africans, and Brazil population because genotype frequencies and prevalence of DN were different among ethnic groups. In order to avoid excessive comparisons, the OR was calculated by using three methodsCitation12,13: method 1, allele comparison (T allele vs. G allele); method 2, comparing TT homozygous with the other two combinations (TT vs. TG + GG); method 3, comparing GG genotype with the other two combinations (GG vs. TG + TT). A χ2-test using a web-based program was applied to determine whether genotype distribution of the control population reported conformed to Hardy–Weinberg equilibrium (HWE) (p < 0.05 was considered significant). Sensitivity analysis was performed when studies with controls were not in HWE. All descriptive data were expressed as mean ± SD.
RESULTS
Study Characteristics
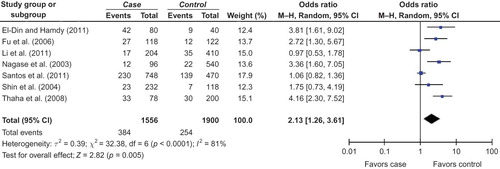
The search yielded 165 references from PubMed, Embase, Cochrane Library, and CBM-disc. According to the inclusion and exclusion criteria, eight articlesCitation14–21 were identified for the analysis of the association between eNOS Glu298Asp gene polymorphism and DN susceptibility in our review (). Five investigationsCitation15–17,20,21 were conducted in Asians, one studyCitation18 for Caucasians, oneCitation14 in Africans, and oneCitation19 in Brazil population. Six studiesCitation14–19 were reported in English and two reportsCitation20,21 were published in Chinese. The data of our interest were extracted and shown in . These eight studies contained 850 case series and 1254 controls. The average frequency of T allele was 19.19% in Asian DN patients and 8.68% in controls. For Caucasians, the frequency of T allele was 52.50% in case group and 22.50% for controls. The frequency of T allele in Brazil population was 30.75% in cases and 29.57% for control group. When compared with that in Brazil population, the ratio of cases/controls for average frequency of T allele was markedly elevated in Asians and Caucasians (Asians, cases/controls = 2.21; Caucasians, cases/controls = 2.33; Brazil population, cases/controls = 1.04).
Table 1. Characteristics of the studies evaluating the effects of eNOS Glu298Asp gene polymorphism on DN risk.
Association of the eNOS Glu298Asp Gene Polymorphisms with DN Risk
In this meta-analysis, a significant association between T allele and DN risk was observed in overall populations ( for T allele; ). Furthermore, the GG genotype might be a protective factor against the risk of DN in overall populations ( for GG allele; ). However, the association of TT genotype with DN risk was not observed ().
Figure 3. The pooled OR indicated that GG genotype was associated with DN risk in overall populations.
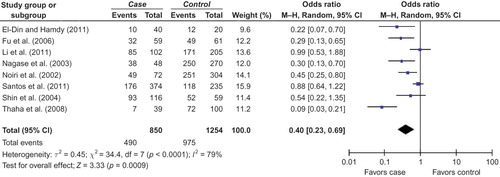
Table 2. Meta-analysis of the association of eNOS Glu298Asp gene polymorphism with the risk of DN.
The ethnical and geopolitical difference might affect the results of our analysis for the association of eNOS Glu298Asp gene polymorphism with DN susceptibility. In order to evaluate this effect, we divided the population by ethnicity. T allele was associated with the risk of DN in Asians and for Caucasians (). Furthermore, GG genotype might play a protective role against DN risk in Asians, Caucasians, and Africans. However, there was no association between TT genotype and DN susceptibility in Asians and Caucasians (). Furthermore, there was no association of eNOS Glu298Asp gene polymorphism with DN susceptibility for Brazil population ().
Sensitivity Analysis
One studyCitation15 from Asians that the genotype distributions in the controls were significantly deviated from HWE was excluded from our sensitive analysis. Furthermore, one studyCitation14 from Africans had not provided the detailed gene distribution data for the control group for HWE test and it was excluded from the sensitive analysis. Finally, four from Asians, one from Caucasians, and one from Brazil population were included for our sensitive analysis.
In the sensitivity analysis for overall populations, we found that the pooled OR for T allele was favorable to DN group (OR = 1.99, 95% CI = 1.13–3.49; ) and the difference was statistically significant (p = 0.02). Furthermore, the pooled OR for GG genotype seemed to play a protective role against DN disease (OR = 0.40, 95% CI = 0.19–0.82; ). Interestingly, TT genotype was not associated with the risk of DN in the sensitivity analysis. The results of the sensitivity analysis were consistent with those of the non-sensitivity analysis for overall populations.
Furthermore, those results of the sensitivity analysis in Asians, Caucasians, and Brazil population were consistent with those of the non-sensitivity analysis ().
DISCUSSION
Damage of endothelial NO generation brought about by gene polymorphism is considered the major deterioration factor for progressive renal disease, such as DN.Citation14 DN is a major health problem associated with very high morbidity and mortality, and data on the risk factors for the pathogenesis of DN were insufficient. There was rare genetic molecular marker to predict the onset of DN. This meta-analysis was performed to explore whether the eNOS Glu298Asp gene polymorphism could predict the susceptibility of DN.
In this investigation, eight suitable studies were recruited into our meta-analysis: five studies from Asians, one investigation for Caucasians, one from Africans, and one from Brazil population. Our meta-analysis showed that T allele was associated with the onset of DN in overall populations, but TT genotype did not. Furthermore, GG genotype seemed to play a protective role against DN risk. Sensitivity analysis was also performed in our meta-analysis, and we found that the results of the sensitivity analysis were similar to those of the non-sensitivity analysis for overall populations. The results for the overall populations might be robust to some extent.
The geographic and ethnic difference might be an important factor to effect the association of gene polymorphism with the susceptibility of DN. In our study, we found that the average frequency of T allele in controls was 22.50% in Caucasians. Furthermore, the frequency of T allele in Brazil population was 29.57% for control group. However, for Asians, the average frequency of T allele for controls was 8.68%. The disequilibrium of T allele distribution in controls was observed among those different races. The subgroup analysis was conducted to explore the association of eNOS Glu298Asp gene polymorphism with the susceptibility of DN in different races.
In Asians, an association between T allele and the risk of DN was found. The ratio of cases/controls for average frequency of T allele was elevated in Asians (cases/controls = 2.21). T allele might be a risk factor to predict the risk of DN in Asians. Furthermore, GG genotype seemed to play a protective role against DN risk in Asians. Interestingly, the results of the sensitivity analysis were consistent with those of the non-sensitivity analysis. T allele might be a risk factor for the risk of DN in Asians.
In Caucasians, there was an association between eNOS Glu298Asp gene polymorphism and susceptibility of DN. However, only one study was recruited into our meta-analysis for Caucasians and it was difficult to draw a robust conclusion for Caucasians. However, more studies should be performed in the future.
GG genotype might be a protective factor against the susceptibility of DN for African population. However, there was only one study recruited for African population in this meta-analysis, and the gene distribution of TT and GT was not provided. The conclusion for African population might be less robust. Whether there was an association between eNOS Glu298Asp gene polymorphism and susceptibility of DN, more religious studies should be performed further.
eNOS Glu298Asp gene polymorphism was not associated with the susceptibility of DN in Brazil population. The gene distributions of the included study were in HWE, and the results of the sensitivity analysis were the same as those of the non-sensitivity analysis in Brazil population. However, only one study was included into our meta-analysis and it was difficult to draw a robust conclusion for Brazil population. More studies in Brazil population should be conducted in the future.
There were some meta-analyses to explore the association of eNOS Glu298Asp gene polymorphism with the susceptibility of some diseases in the past years. Su et al.Citation22 performed a meta-analysis to investigate the association of eNOS Glu298Asp gene polymorphism with recurrent pregnancy loss (RPL) and reported that eNOS Glu298Asp gene polymorphism was significantly associated with RPL. Casas et al.Citation23 performed a meta-analysis to explore the relationship between eNOS genotype and ischemic heart disease (IHD) and reported that homozygosity for the TT was associated with an increased risk of ischemic heart disease Shaik et al.Citation24 reported that eNOS Glu298Asp gene polymorphism was not associated with the risk of preeclampsia in women by meta-analysis method. Yu et al.Citation25 conducted a meta-analysis to study the relationship between eNOS Glu298Asp polymorphism and preeclampsia risk and found that the eNOS Glu298Asp polymorphism was not associated with a significant increased risk of preeclampsia in overall populations, Caucasians, and Asians. In this meta-analysis, the relationship between eNOS Glu298Asp gene polymorphism and DN risk was explored and our results were similar to those of Su et al.Citation22
In our investigation, we found that the T allele was associated with DN susceptibility in overall populations, Asians, and Caucasians, and GG genotype might play a protective role against DN susceptibility in overall populations, Asians, Caucasians, and Africans. However, the association was not found in Brazil population. These findings should be regarded cautiously because many other ingredients, such as heterogeneity of enrolled cases, limited statistical power, variable study designs, and different interventions, were closely related to affect the results. Furthermore, the gene polymorphisms of the eNOS 894G>T and −786T>C were also reported to be associated with the developing DN patients.Citation26 Whether the eNOS Glu298Asp gene polymorphism is just linked with other discrete loci involved in the occurrence of DN is not clear at the moment. Undoubtedly, the limitations mentioned above might affect our final conclusions.
In conclusion, the results of our study support that T allele was associated with DN susceptibility in overall populations, Asians, and Caucasians, and GG genotype might play a protective role against DN susceptibility in overall populations, for Asians, in Caucasians, and in Africans. However, more case–control association investigations on larger, stratified populations are required to further clarify the role of this eNOS Glu298Asp gene polymorphism in DN susceptibility in different ethnicities.
ACKNOWLEDGMENT
We gratefully acknowledge the most helpful comments on this article received from Professor Liang Rong, Department of Pediatric-neonatology, Baylor College of Medicine, Houston, TX, USA.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Lee AS, Lee YJ, Lee SM, . An aqueous extract of Portulaca oleracea ameliorates diabetic nephropathy through suppression of renal fibrosis and inflammation in diabetic db/db mice. Am J Chin Med. 2012;40:495–510.
- Palmer ND, Freedman BI. Insights into the genetic architecture of diabetic nephropathy. Curr Diab Rep. 2012;12:423–431.
- Yu ZY, Chen LS, Zhang LC, Zhou TB. Meta-analysis of the relationship between ACE I/D gene polymorphism and end-stage renal disease in patients with diabetic nephropathy. Nephrology (Carlton). 2012;17:480–487.
- Reis KA, Ebinc FA, Koc E, . Association of the angiotensinogen M235T and APO E gene polymorphisms in Turkish type 2 diabetic patients with and without nephropathy. Ren Fail. 2011;33:469–474.
- Datta SK, Kumar V, Pathak R, . Association of glutathione S-transferase M1 and T1 gene polymorphism with oxidative stress in diabetic and nondiabetic chronic kidney disease. Ren Fail. 2010;32:1189–1195.
- Larsen T, Mose FH, Bech JN, Pedersen EB. Effect of nitric oxide inhibition on blood pressure and renal sodium handling: A dose– response study in healthy man. Clin Exp Hypertens. 2012. doi:10.3109/10641963.2012.681727.
- Schwartz IF, Grupper A, Soetendorp H, . Attenuated glomerular arginine transport prevents hyperfiltration and induces HIF-1alpha in the pregnant uremic rat. Am J Physiol Renal Physiol. 2012;303:F396–F404.
- Tsai KD, Chang WW, Lin CC, . Differential effects of LY294002 and wortmannin on inducible nitric oxide synthase expression in glomerular mesangial cells. Int Immunopharmacol. 2012;12:471–480.
- Zhou TB, Qin YH, Su LN, . Insertion/deletion (I/D) polymorphism of angiotensin-converting enzyme gene in steroid-resistant nephrotic syndrome for children: A genetic association study and meta-analysis. Ren Fail. 2011;33:741–748.
- Zhou TB, Qin YH, Su LN, . The association between angiotensin-converting enzyme insertion/deletion gene variant and risk of focal segmental glomerulosclerosis: A systematic review and meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011;12:624–633.
- Zhou TB, Ou C, Qin YH, . Association of angiotensin converting enzyme insertion/deletion gene polymorphism with idiopathic nephrotic syndrome susceptibility in children: A meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011;12:601–610.
- Zhou TB, Qin YH, Su LN, Lei FY, Huang WF, Zhao YJ. ACE I/D gene polymorphism can’t predict the steroid responsiveness in Asian children with idiopathic nephrotic syndrome: A meta-analysis. PLoS One. 2011;6:e19599.
- Zhou TB, Liu YG, Lin N, . Relationship between angiotensin-converting enzyme insertion/deletion gene polymorphism and systemic Lupus Erythematosus/Lupus Nephritis: A systematic review and metaanalysis. J Rheumatol. 2012;39:686–693.
- Noiri E, Satoh H, Taguchi J, . Association of eNOS Glu298Asp polymorphism with end-stage renal disease. Hypertension. 2002;40:535–540.
- Nagase S, Suzuki H, Wang Y, . Association of ecNOS gene polymorphisms with end stage renal diseases. Mol Cell Biochem. 2003;244:113–118.
- Shin SY, Baek SH, Chang KY, . Relations between eNOS Glu298Asp polymorphism and progression of diabetic nephropathy. Diabetes Res Clin Pract. 2004;65:257–265.
- Thaha M, Pranawa, Yogiantoro M, . Association of endothelial nitric oxide synthase Glu298Asp polymorphism with end-stage renal disease. Clin Nephrol. 2008;70:144–154.
- El-Din BS, Hamdy SM. Impact of nitric oxide synthase Glu298Asp polymorphism on the development of end-stage renal disease in type 2 diabetic Egyptian patients. Ren Fail. 2011;33:878–884.
- Santos KG, Crispim D, Canani LH, Ferrugem PT, Gross JL, Roisenberg I. Association of eNOS gene polymorphisms with renal disease in Caucasians with type 2 diabetes. Diabetes Res Clin Pract. 2011;91:353–362.
- Fu Z, Li C, Xiao Z, Wang Z. The relationship between chromosome 7q35 region gene mutation and diabetic nephropathy. Chin J Nephrol. 2006;22:59.
- Li X, Yu M, Wu X, Shui H, Xiao J. Assodatiml of endothelial nitric oxide synthase gene Glu298Asp polymorphism with diabetic kidney disease. J Clin Nephrol. 2011;11:351–353.
- Su MT, Lin SH, Chen YC. Genetic association studies of angiogenesis- and vasoconstriction-related genes in women with recurrent pregnancy loss: A systematic review and meta-analysis. Hum Reprod Update. 2011;17:803–812.
- Casas JP, Bautista LE, Humphries SE, Hingorani AD. Endothelial nitric oxide synthase genotype and ischemic heart disease: Meta-analysis of 26 studies involving 23028 subjects. Circulation. 2004;109:1359–1365.
- Shaik AP, Sultana A, Bammidi VK, Sampathirao K, Jamil K. A meta-analysis of eNOS and ACE gene polymorphisms and risk of pre-eclampsia in women. J Obstet Gynaecol. 2011;31:603–607.
- Yu CK, Casas JP, Savvidou MD, Sahemey MK, Nicolaides KH, Hingorani AD. Endothelial nitric oxide synthase gene polymorphism (Glu298Asp) and development of pre-eclampsia: A case– control study and a meta-analysis. BMC Pregnancy Childbirth. 2006;6:7.
- Shoukry A, Shalaby SM, Abdelazim S, . Endothelial nitric oxide synthase gene polymorphisms and the risk of diabetic nephropathy in type 2 diabetes mellitus. Genet Test Mol Biomarkers. 2012;16:574–579.