Abstract
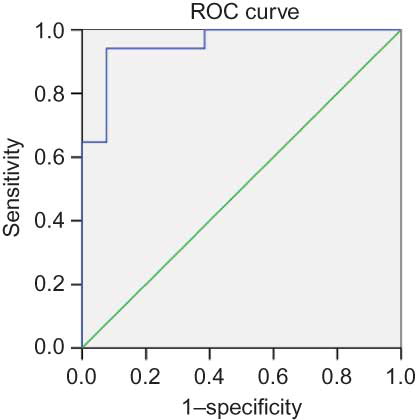
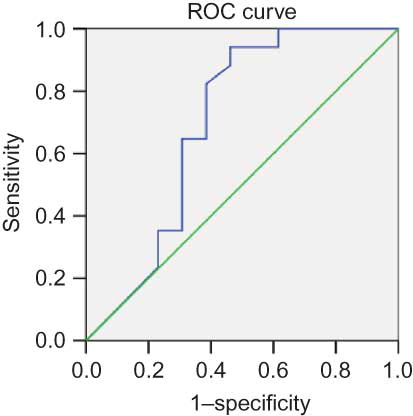
Background: To determine predictors of survival in patients with microscopic polyangiitis (MPA). Methods: A cohort of 64 patients who met the Chapel Hill criteria for MPA with renal involvement participated in the study. All subjects received cytotoxic drugs. All of the diagnoses were biopsy proven. Results: We retrospectively studied 64 patients (median age, 59 years; male/female ratio, 1.6:1). The mean follow-up was 38 months; 34 (53.13%) patients died or acquired end-stage renal disease. According to univariate analysis, a preliminary prognostic value was attributed to serum creatinine (Scr) > 459 μmol/L (p < 0.001); erythrocyte sedimentation rate (ESR) > 99 mm/h (p < 0.001); serum albumin < 30 g/L (p < 0.001); and hemoglobin < 84 g/L (p < 0.001). Logistic regression analysis showed that Scr level (β = 1.02, p = 0.0002) and ESR (β = 1.02, p = 0.0002) at baseline were associated with poor prognosis, and Cox regression analysis further confirmed this result [Scr: β = 1.004, 95% confidence interval (CI): 1.002–1.006, p < 0.001; ESR: β = 1.018, 95% CI: 1.000–1.037, p = 0.046]. The receiver operating characteristic curve showed that Scr and ESR were predictors of MPA patient prognosis, their areas under the curves were 0.95 and 0.80, their sensitivities were 94.1% and 92.3%, and their specificities were 94% and 70%, respectively. Conclusion: Despite the small number of patients in this study, the prevalence of renal vasculitis was high in patients with MPA. The level of Scr and ESR may be a useful clinical biomarker for monitoring prognosis.
INTRODUCTION
Pauci-immune vasculitis is a subtype of entities defined as rapidly progressive glomerulonephritis (RPGN), which is characterized by the presence of glomerular crescents. Approximately 80% are the cases of anti-neutrophil cytoplasmic antibody (ANCA)-associated pauci-immune vasculitides, and they are the most frequent cause of RPGN in adults.Citation1
These primary small-vessel vasculitides are a group of life-threatening diseases,Citation2 including Wegener’s granulomatosis (WG), Churg–Strauss syndrome (CSS), and microscopic polyangiitis (MPA).Citation3,4 Renal vasculitis is the most common severe manifestation of MPA, occurring in more than 50% of cases during the course of the disease.Citation5 Little is known about the prognostic factors and disease activity assessment in MPA cases with renal involvement. Several authors have proposed impaired renal function as a predictor of poor outcome.Citation6,7
The aim of this study was to identify factors that were predictive of survival in 64 patients who were newly diagnosed with MPA in the renal division through prospective follow-up. We also attempted to correlate patient survival with initial disease activity, as assessed by the erythrocyte sedimentation rate (ESR), serum creatinine (Scr), and the Birmingham vasculitis activity score (BVAS).
PATIENTS AND METHODS
Inclusion and Exclusion Criteria
All patients who were newly diagnosed with MPA with renal vasculitis between 1 January 2006 and 31 December 2008 in the Renal Division of Renji Hospital were included. A total of 64 patients with MPA were enrolled in this study. All of the patients in the study had undergone renal biopsy. All of the biopsies were found to be suitable for a definitive diagnosis and were reviewed by two independent pathologists.
Patients with myeloperoxidase (MPO)-ANCA or proteinase-3 (PR3)-ANCA positivity and renal involvement, as evidenced by necrotizing glomerulonephritis on biopsy or red cell casts or hematuria (≥30 red cells per high-power field) on urinalysis,Citation8 were included in the study. All of the subjects were over 18 years old.
Patients with other small-vessel angiitides, such as WG, malignancy-associated vasculitis, or connective tissue disease-associated vasculitis, were excluded from the study.
Methods
Age and sex were recorded at diagnosis for every patient. In addition, the following laboratory parameters were assessed: ESR, C-reactive protein (CRP), Scr, white blood cell count, neutrophil count, lymphocyte count, hemoglobin (Hb) level, platelet count, albuminemia, proteinuria, lipid profile, blood pressure, and ANCA. The BVASCitation9 was calculated at diagnosis.
ANCA tests were performed using both an indirect immunofluorescence (IIF) assay and an antigen-specific enzyme-linked immunosorbent assay (ELISA) for all patients at the time of presentation and before the institution of immunosuppressive treatment. A standard IIF assay was performed according to the manufacturer’s instructions (EUROIMMUN, Lübeck, Germany). In antigen-specific ELISA, two ANCA antigens, PR3 and MPO, which were highly purified as previously reported,Citation10 were used in solid-phase assays.
Treatment
The treatment protocols have been described previously.Citation11,12 The induction therapy included corticosteroids in combination with cyclophosphamide (CTX). Oral prednisone was prescribed at an initial dosage of 1 mg/kg/day for 4–6 weeks, and doses were reduced over time to 12.5–15 mg by 3 months. Intravenous CTX (0.5–1.0 g/m2 every month) was started 10–14 days following the institution of corticosteroids. A 25% dose reduction of CTX was performed for patients over 65 years old, patients who developed leukocytopenia (<4000 cells/mm3), and patients with renal insufficiency. Patients with acute renal failure or pulmonary hemorrhage received three pulses of intravenous methylprednisolone (7–15 mg/kg/day) before the standard induction therapy. Patients with severe pulmonary hemorrhage additionally received plasma exchanges. For maintenance therapy, intravenous CTX was administered every 3 months.
RENAL RESPONSE TO TREATMENT
The renal response to treatment at 6 months after the initiation of immunosuppressive therapy was judged according to the following criteria: (i) complete recovery of renal function, which was indicated by the normalization of renal function and resolution of hematuria; (ii) partial recovery of renal function, which was indicated by stabilization or improvement of renal function, with Scr ≥133 μmol/L but dialysis independent; and (iii) treatment failure, which was indicated by a progressive decline in kidney function with the persistence of active urinary sediment despite immunosuppressive therapy.
The primary end point of the study was all-cause mortality and end-stage renal disease (ESRD) (or doubled Scr). All of the patients were divided into two groups: group A did not reach the primary end point, whereas group B reached the primary end point. Clinical parameters and medication were compared between the two groups. Information on the patients was gathered during the study period through their medical charts or by telephone contact with the treating physicians, the patients, or their family members.
STATISTICAL ANALYSIS
Quantitative variables were compared using Student’s t-test or a nonparametric test, and qualitative variables were compared with the chi-square test or, when appropriate, Fisher’s exact test. Patient survival was assessed by life-table analysis using the Kaplan–Meier method. Survival was evaluated as a function of the parameters recorded at diagnosis, and Cox proportional hazards modelsCitation13 were fitted to examine their individual and combined effects. A p-value <0.05 was considered to be statistically significant. The data were analyzed using SPSS version 13 (SPSS Inc., Chicago, IL, USA).
RESULTS
Sixty-four patients with a new diagnosis of MPA with renal involvement were enrolled in the study. The median age at presentation was 59.87 ± 1.98 years. The principal demographics and clinical, biologic, and immunologic data at diagnosis are summarized in Table 1. The median duration of follow-up was 38 (21–55) months. During the follow-up period, 8 patients (8 of 64, 12.5%) died and 26 (26 of 64, 40.63%) patients developed ESRD. The main cause of death was infection and cardiovascular disease, including myocardial infarction and cerebrovascular accident.
Table 1. Clinical background of all patients (mean ± SD).
The most common pathologic diagnosis was crescentic glomerulonephritis, which was observed in 50% of all patients (). Focal segmental crescent formation was observed in 24 patients (37.5%), and mesangial proliferative glomerulonephritis and focal segmental glomerulosclerosis (12.5%) accounted for the remaining pathologic diagnoses. In our study, 37 (58%) patients were MPO-ANCA positive, and 27 (42%) were PR3-ANCA positive.
A comparison of the characteristics of participants who did and did not reach the primary end point is shown in . There was no significant difference in age, gender, or blood pressure between the two groups. However, compared with group B, the patients in group A had lower creatinine (623.9 ± 245.51 μmol/L vs. 244.7 ± 126.71 μmol/L, p < 0.01). shows that patients in group B tended to have a higher ESR (112.06 ± 24.04 vs. 76.38 ± 48.45 mm/h, p < 0.01).The concentration of albumin in group B patients (29.43 ± 3.08 g/dL) was lower than that of group A patients (31.57 ± 4.21 g/dL, p < 0.01). There was no significant difference in urinary protein excretion, lipid profile, ANCA titer, or BVAS between the two groups.
Table 2. Comparison of patients reaching the primary end point.Footnotea
also shows that Hb levels were lower in group B patients compared with group A patients. However, we did not find a difference in white blood cell, neutrophil, lymphocyte, or platelet counts between the two groups. Patients who reached the end point tended to have a higher level of high-sensitivity C-reactive protein (26.57 ± 7.92 vs. 37.28 ± 5.29 mg/dL), although the difference was not statistically significant ().
We further performed a logistic regression analysis of predictors that were associated with poor prognosis (). A higher Scr level [odds ratio (OR) = 1.01, 95% confidence interval (CI): 1.007–1.024, p < 0.01], higher ESR (OR = 1.03, 95% CI: 1.009–1.042, p < 0.01), lower serum albumin (sALB) level (OR = 0.85, 95% CI: 0.726–0.986, p < 0.05), and lower Hb (OR = 0.96, 95% CI: 0.934–0.989, p < 0.01) were found to be significantly associated with shorter survival. Cox regression analysis further confirmed this result (Scr: β = 1.004, 95% CI: 1.002–1.006, p < 0.001; ESR: β = 1.018, 95% CI: 1.000–1.037, p = 0.046) ().
Table 3. Logistic regression analysis of outcome.
Table 4. Multivariate analysis of factors associated with survival in 64 patients using a Cox proportional hazards regression model.
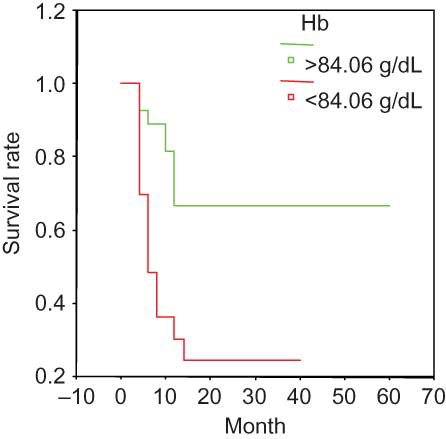
The Kaplan–Meier survival curve suggested the following parameters as predictors of poor prognosis: Scr > 459 μmol/L (); ESR > 99 mm/h (); Hb < 84.06 g/L (); and sALB < 30 g/L (). None of the other analyzed variables were significantly associated with survival.
Figure 1. Survival and serum creatinine in the entire group. Scr > 459.73 umol/L at presentation was associated with a significantly poor prognosis.
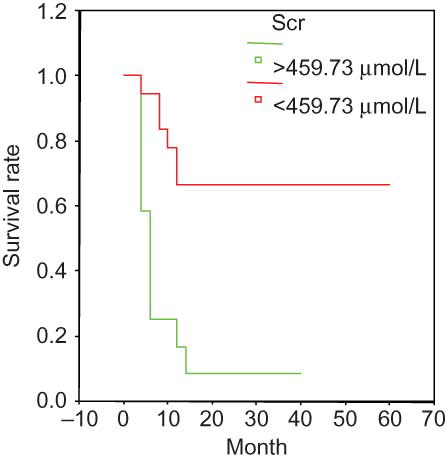
Figure 2. Survival and ESR in the entire group. ESR > 99.12 mm/h at presentation was associated with a significantly poor prognosis.
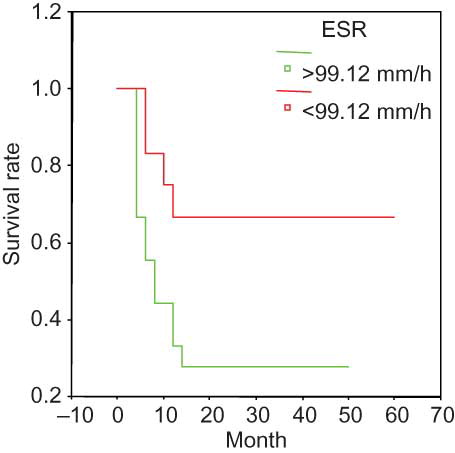
Figure 3. Survival and serum albumin in the entire group. sALB < 30.04 g/dL at presentation was associated with a significantly poor prognosis.
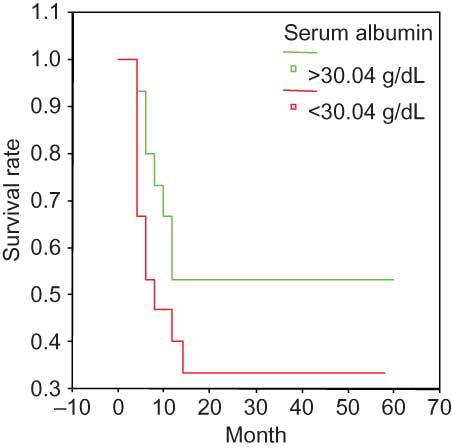
Figure 4. Survival and hemoglobin in the entire group. Hb < 84.06 g/dL at presentation was associated with a significantly poor prognosis.
We evaluated the specificity and sensitivity of clinical parameters for the prediction of poor prognosis ( and B). The Scr level showed high sensitivity (94.1%) and specificity (92.3%); the receiver operating characteristic (ROC) area under the curve was 0.94. The sensitivity and specificity of ESR were 94% and 70%, respectively, and the ROC was 0.8.
DISCUSSION
Renal involvement is frequently present in ANCA-associated systemic vasculitis and is an important cause of ESRD. This study analyzed predictors of survival in 64 patients who were newly diagnosed with MPA through prospective follow-up. Despite immunosuppressive treatments, some patients still had severe complications, and up to 40% of patients required replacement renal therapy in our study. The overall mortality rate in our study was higher than that previously reported by other authors.Citation14
The predictors of outcome for MPA with renal involvement are highlighted in this study. We conclude that the following parameters, when present at diagnosis, are independent predictors of the primary end point: impaired renal function, with a Scr threshold value of 459 μmol/L, and high ESR, with a threshold value of 90 mm/h. Furthermore, the significant association between Hb, sALB, and mortality suggests that they could also be the markers of prognosis in MPA. No relationship was established between any of the other demographic, clinical, or biological parameters and patient survival.
Systemic vasculitis was more common in older age groups. The demographic data in our study did not show a relationship between age and survival, although a previous study found that an age of >57 years at diagnosis was a marker of poor prognosis.Citation15 Clinicians treating older patients should maintain a high index of suspicion regarding the diagnosis of ANCA-associated vasculitis as these patients have reduced renal reserve and are more likely to present with severe renal disease.Citation16 In our study, the average age of the patients was 59.87 years; therefore, it appears that the patients were younger than the previous study, and we found no association with survival. Consistent with previous reports, there was no significant sex difference.Citation17
The outcomes of MPA were related to the creatinine level at presentation; thus, diagnostic delay may have a major influence on outcome. An increased awareness of MPA and recognition of the presence of renal involvement at diagnosis should shorten the delay in diagnosis. Urine dipstick analysis provides a simple bedside test to identify early renal pathological states. Furthermore, the combination of proteinuria, microscopic hematuria, and a positive ANCA result has up to a 95% positive predictive value for the presence of necrotizing glomerulonephritis.Citation18 In our study, the most common pathologic diagnosis was crescentic glomerulonephritis, which was observed in 50% of all MPA cases. Focal segmental crescent formation was observed in 24 patients (37.5%), and glomerulonephritis and focal segmental glomerulosclerosis were observed in 12.5%. Renal disease has been suggested as a predictor of poor outcome in ANCA-associated vasculitis. The impact of kidney involvement on prognosis was confirmed by Luqmani et al.,Citation19 who noted that patients with Scr > 5.65 mg/dL had a poor outcome. In our study, the mean Scr concentration was 459 μmol/L, which was lower than the previously reported thresholds.Citation15 These findings suggest that prognosis might depend on the deterioration of renal function. Similar findings were reported by other investigators.Citation20,21
In this study, patients who reached the end point had significantly lower mean Hb and sALB levels and considerably higher acute-phase parameters (ESR and CRP levels) than the group with a better prognosis. However, no significant differences were noted in neutrophil count, lymphocyte count, or platelet count between the two groups. We also found a strong association between ESR at diagnosis and patient survival. This finding suggests that ESR could be a good marker of disease severity in MPA. In contrast, we did not establish a relationship between ANCA titers and mortality. We therefore believe that the intrinsic activity of MPA might be more accurately reflected by Scr level and ESR. Similarly, disease activity assessment with BVAS was not statistically correlated with survival. This result is consistent with previous findings, as BVAS relies primarily on the cumulative evaluation of the anatomical extent of the disease.Citation22 In another study,Citation23 the author thought BVAS was calculated retrospectively, which can lead to its underestimation because of the numerous parameters it takes into consideration, but this did not affect the results, as any oversight would merely have increased the score. Therefore, on the basis of our results, disease activity scoring with BVAS does not seem to be appropriate for the assessment of prognosis in MPA.
Our relapse rate was lower compared with previous series. This difference may be explained by the limited number of patients and the absence of WG in our group. Weidner et al.Citation15 reported six relapses in WG patients. Lionaki et al.Citation18 reported a low relapse rate in perinuclear ANCA-positive patients, similar to our series. The relapse rate of pauci-immune vasculitis among patients on chronic dialysis was lower compared with patients with preserved renal function.
In conclusion, our survival analysis suggests that Scr > 459 μmol/L, ESR > 99 mm/h, sALB < 30 g/dL, and Hb < 84 g/L at the time of diagnosis are independent predictors of poor prognosis for patients with MPA.
ACKNOWLEDGMENTS
We thank all of the nephrologists in the Renal Division, Renji Hospital, Shanghai Jiao Tong University School of Medicine, for their work.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
This work was supported by the National Basic Research Program of China 973 Program No. 2012CB517600 (Grant No. 2012CB517602); the National Key Technology Research and Development (R&D) Program of the Ministry of Science and Technology of China (Grant No. 2011BAI10B04); the National Natural Science Foundation of China (Grant No. 81102700); the Science and Technology Commission of Shanghai Municipality (Grant Nos. 09dZ1973600 and 10JC1410100); and the Shanghai Municipal Health Bureau (Grant No. 2010L063A).
REFERENCES
- Jeanette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164–1177.
- Savage CO, Harper L, Holland M. New findings in pathogenesis of antineutrophil cytoplasm antibody-associated vasculitis. Curr Opin Rheumatol. 2002;14:15–22.
- Seo P, Stone JH. The antineutrophil cytoplasmic antibody-associated vasculitides. Am J Med. 2004;117:39–50.
- Chen M, Yu F, Wang SX, . Antineutrophil cytoplasmic autoantibody-negative pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol. 2007;18:599–605.
- Weidanz F, Day CJ, Hewins P, . Recurrences and infections during continuous immunosuppressive therapy after beginning dialysis in ANCA-associated vasculitis. Am J Kidney Dis. 2007;50:36–46.
- Hoffman GS, Kerr GS, Leavitt RY, . Wegener’s granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–498.
- Gera M, Griffin MD, Specks U, . Recurrence of ANCA-associated vasculitis following renal transplantation in the modern era of immunosuppression. Kidney Int. 2007;71:1296–1301.
- RAVE-ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232.
- Mukhtyar C, Lee R, Brown D, . Modification and validation of the Birmingham vasculitis activity score (version 3). Ann Rheum Dis. 2009;68:1827–1832.
- Hagen C, Daha MR, Hermans J, . Diagnostic value of standardized assays for antineutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. Kidney Int. 1998;53:747–753.
- De Groot K, Harper L, Jayne DRW, . Pulse versus daily oral cyclophosphamide or induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–680.
- Jayne D, Rasmussen N, Andrassy K, . A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44.
- Cox DR. Regression models and life table tables. J R Stat Soc. 1972;34:187–219.
- Hogan SL, Nachman PH, Wilkman AS, . Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:23–32.
- Weidner S, Geuss S, Hafezi-Rachti S, . ANCA-associated vasculitis with renal involvement: an outcome analysis. Nephrol Dial Transplant. 2004;19:1403–1411.
- Harper L, Savage CO. ANCA-associated renal vasculitis at the end of the twentieth century—a disease of older patients. Rheumatology. 2005;44(4):495–501.
- Vassallo M, Shepherd RJ, Iqbaal P, . Age-related variations in presentation and outcome in Wegener’s granulomatosis. J R Coll Physicians Lond. 1997;31:396–400.
- Lionaki S, Hogan SL, Jennette CE, . The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int. 2009;76:644–651.
- Luqmani RA, Bacon PA, Beaman M, . Classical versus non-renal Wegener’s granulomatosis. Q J Med. 1994;87:161–167.
- Merino JL, Galeano C, Espejo B, . A retrospective study on outcome of microscopic polyangiitis in chronic renal replacement therapy. Nephrol Dial Transplant. 2011;26(4):1360–1366.
- Itabashi M, Takei T, Yabuki Y, . Clinical outcome and prognosis of anti-neutrophil cytoplasmic antibody-associated vasculitis in Japan. Nephron Clin Pract. 2010;115(1):c21–c27.
- Mahr A, Girard T, Agher R, . Analysis of factors predictive of survival based on 49 patients with systemic Wegener’s granulomatosis and prospective follow-up. Rheumatology (Oxford). 2001;40:492–498.
- Cruz BA, Ramanoelina J, Mahr A, . Prognosis and outcome of 26 patients with systemic necrotizing vasculitis admitted to the intensive care unit. Rheumatology (Oxford). 2003;42(10):1183–1188.